SAE0044
Choline Oxidase from Arthrobacter sp.
recombinant, expressed in E. coli ≥12 units/mg protein
Synonym(s):
COD, COX, ChOx, Choline: oxygen 1-oxidoreductase
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Recommended Products
biological source
bacterial (arthrobacter sp.)
Quality Level
recombinant
expressed in E. coli ≥12 units/mg protein
form
powder
specific activity
≥12 units/mg protein
technique(s)
activity assay: suitable
UniProt accession no.
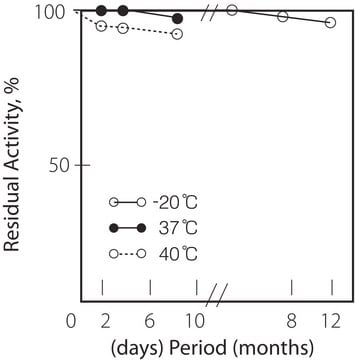
storage temp.
−20°C
General description
Research area: Cell Signaling
Choline oxidase from Arthrobacter sp. is a flavoprotein, which is a member of the glucose–methanol–choline (GMC). The codA gene encodes the choline oxidase enzyme.
Choline oxidase from Arthrobacter sp. is a flavoprotein, which is a member of the glucose–methanol–choline (GMC). The codA gene encodes the choline oxidase enzyme.
Application
Choline oxidase from Arthrobacter sp. has been used in the enzymatic determination of choline in milk using a flow injection analysis (FIA) system with potentiometric detection. It has been used for fluorescent detection of choline released during autotaxin (ATX) activity in conditioned media and plasma.
Biochem/physiol Actions
Choline oxidase from Arthrobacter sp. is a flavoprotein, which is a member of the GMC-oxidoreductase family. The enzyme catalyzes the four-electron-oxidation of choline to glycine betaine via the intermediate betaine aldehyde, in two sequential FAD-dependent reaction steps. The enzyme is useful for enzymatic determination of phosphatidylcholine by coupling with phospholipase D or determination of sphingomyelin by coupling with sphingomyelinase and for cholinesterase activity assays.Choline oxidase (COD) mediating glycine betaine biosynthetic pathway plays a vital role in creating stress resistant transgenic plants.
Unit Definition
One unit will form 1 μmole of H2O2 with oxidation of 1 μmole of choline to betaine aldehyde per min at pH 8.0 at 37 °C. Note: During the conversion of choline to betaine by choline oxidase, 2 μmoles of H2O2 are produced for every μmole of choline.
Preparation Note
It is recommended to reconstitute choline oxidase in phosphate buffer at pH 7.5 supplemented with 10mM EDTA. Reconstituted material can be stored at 2-8 °C for at least 2 weeks.
Storage and Stability
Tightly closed.Dry. Keep locked up or in an area accessible only to qualified or authorized
persons
persons
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Transformation of Arabidopsis thaliana with the codA gene for choline oxidase; accumulation of glycinebetaine and enhanced tolerance to salt and cold stress.
Hayashi H, et al.
The Plant Journal, 12(1), 133-142 (1997)
Transformation of Synechococcus with a gene for choline oxidase enhances tolerance to salt stress.
Deshnium P, et al.
Plant Molecular Biology, 29(5), 897-907 (1995)
The use of bacterial choline oxidase, a glycinebetaine-synthesizing enzyme, to create stress-resistant transgenic plants
Plant Physiology, 180-188 (2001)
J L Lima et al.
The Analyst, 125(7), 1281-1284 (2000-09-14)
The development of a FIA system for the determination of total choline content in several types of milk is described. The samples were submitted to hydrochloric acid digestion before injection into the system and passed through an enzymatic reactor containing
New enzymatic assay of cholinesterase activity
Okabe, H. et al.,
Clinica Chimica Acta; International Journal of Clinical Chemistry, 80, 87-94 (1977)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service