19533
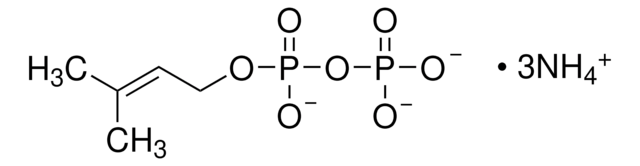
Geranyl pyrophosphate lithium salt
analytical standard
Synonym(s):
(E)-3,7-Dimethyl-2,6-octadien-1-ol trihydrogen pyrophosphate lithium salt, (E)-3,7-Dimethyl-2,6-octadien-1-yl pyrophosphate lithium salt, GPP-Li
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C10H20O7P2 · xLi+
CAS Number:
Molecular Weight:
314.21 (free acid basis)
Beilstein:
1915690
UNSPSC Code:
85151701
NACRES:
NA.24
Recommended Products
Quality Level
grade
analytical standard
Assay
≥95.0% (HPLC)
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
food and beverages
format
neat
shipped in
dry ice
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
Application
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Analysis Note
NMR-Internal Standard : KHP
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Charles Burke et al.
The Journal of biological chemistry, 277(5), 3141-3149 (2001-12-26)
Geranyl diphosphate synthase belongs to a subgroup of prenyltransferases, including farnesyl diphosphate synthase and geranylgeranyl diphosphate synthase, that catalyzes the specific formation, from C(5) units, of the respective C(10), C(15), and C(20) precursors of monoterpenes, sesquiterpenes, and diterpenes. Unlike farnesyl
Orapin Ariyawutthiphan et al.
Acta crystallographica. Section D, Biological crystallography, 68(Pt 11), 1558-1569 (2012-10-24)
In the typical isoprenoid-biosynthesis pathway, condensation of the universal C(5)-unit precursors isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) occurs via the common intermediates prenyl pyrophosphates (C(10)-C(20)). The diversity of isoprenoids reflects differences in chain length, cyclization and further additional modification
A Montalvetti et al.
The Journal of biological chemistry, 276(36), 33930-33937 (2001-07-04)
We report the cloning and sequencing of a gene encoding the farnesyl pyrophosphate synthase of Trypanosoma cruzi. The protein (T. cruzi farnesyl pyrophosphate synthase, TcFPPS) is an attractive target for drug development, since the growth of T. cruzi is inhibited
Sarah A Holstein et al.
Lipids, 39(4), 293-309 (2004-09-11)
The isoprenoid biosynthetic pathway is the source of a wide array of products. The pathway has been highly conserved throughout evolution, and isoprenoids are some of the most ancient biomolecules ever identified, playing key roles in many life forms. In
Joris W De Schutter et al.
Bioorganic & medicinal chemistry letters, 20(19), 5781-5786 (2010-08-31)
A structure-based approach was pursued in designing novel bisphosphonate inhibitors of the human farnesyl pyrophosphate synthase (hFPPS). Preliminary SAR and structural evidence for the simultaneous binding of these inhibitors into the isopentenyl pyrophosphate (IPP) and the geranyl pyrophosphate (GPP) substrate
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service










![Guanosine 5′-[β,γ-imido]triphosphate trisodium salt hydrate ≥85% (HPLC), powder](/deepweb/assets/sigmaaldrich/product/structures/204/494/05808804-1ca7-44bf-b6c5-d4934dc7cb85/640/05808804-1ca7-44bf-b6c5-d4934dc7cb85.png)