851450
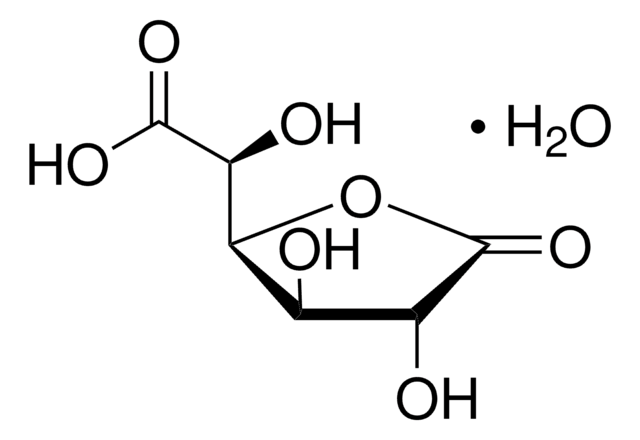
D-(+)-Glucuronic acid γ-lactone
≥99%
Synonym(s):
D-(+)-Glucurono-6,3-lactone, D-Glucurone, D-Glucurono-6,3-lactone, Glucuronolactone
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C6H8O6
CAS Number:
Molecular Weight:
176.12
Beilstein:
83595
EC Number:
MDL number:
UNSPSC Code:
12352115
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥99%
form
powder
optical activity
[α]24/D +18.8°, c = 8 in H2O
mp
172-175 °C (lit.)
solubility
water: soluble 25 mg/mL, clear, colorless
SMILES string
O=C([C@@H]([C@@H](O1)[C@H](O)[C@H](O)C1=O)O)[H]
InChI
1S/C6H8O6/c7-1-2(8)5-3(9)4(10)6(11)12-5/h1-5,8-10H/t2-,3+,4-,5+/m0/s1
InChI key
UYUXSRADSPPKRZ-SKNVOMKLSA-N
Looking for similar products? Visit Product Comparison Guide
General description
D-(+)-Glucuronic acid γ-lactone (Glucourono-γ-lactone, Glucurone or Glycurone) is a carbohydrate derivative. It converted into L-ascorbic acid in animals and human body. Its molecule contains two five-membered rings. Its crystal structure has been studied.
Application
D-(+)-Glucuronic acid γ-lactone may be used in the following studies:
- As starting ragent in the synthesis of 2,3,4,-tris(tert.-butyldimethysilyl) glucuronic acid trichloroethylester, required for the preparation of 1-O-acyl glucuronide of the anti-inflammatory drug ML-3000.
- Synthesis of optically active glucopyranoses.
- Synthesis of long-chain alkyl glucofuranosides.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Matthew I Worthley et al.
The American journal of medicine, 123(2), 184-187 (2010-01-28)
Energy drink consumption has been anecdotally linked with sudden cardiac death and, more recently, myocardial infarction. As myocardial infarction is strongly associated with both platelet and endothelial dysfunction, we tested the hypothesis that energy drink consumption alters platelet and endothelial
J M Hsu et al.
The Journal of nutrition, 111(1), 141-145 (1981-01-01)
The effect of feeding 0.25% ethionine for 3-10 weeks to male and female rats on urinary and tissue ascorbate contents were studied. The concentrations of ascorbic acid in the urine, blood, liver and adrenals were significantly reduced in the rats
Chemical & Pharmaceutical Bulletin, 41, 1197-1197 (1993)
W C Koller et al.
Neurology, 42(9), 1807-1808 (1992-09-01)
The metabolism of oral glucurolactone to serum D-glucarate by the hepatic cytochrome P450 enzyme system was no different in 20 untreated Parkinson's disease (PD) patients as compared with 20 age- and sex-matched controls. There was no evidence for a deficit
J A Horne et al.
Amino acids, 20(1), 83-89 (2001-04-20)
500 ml of a glucose based "energy" drink versus a control without the active ingredients (caffeine, taurine, glucuronolactone) were given double blind to 11 sleepy participants driving an interactive real-car driving simulator. Lane drifting and a secondary task (reaction time)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service