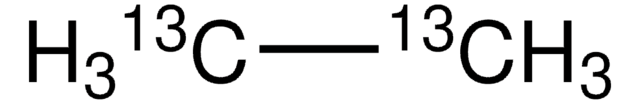All Photos(1)
About This Item
Linear Formula:
CH3CH3
CAS Number:
Molecular Weight:
30.07
Beilstein:
1730716
EC Number:
MDL number:
UNSPSC Code:
12142100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
1.05 (vs air)
Quality Level
vapor pressure
37.95 atm ( 21.1 °C)
Assay
99.99%
form
gas
autoignition temp.
881 °F
expl. lim.
13 %
bp
−88 °C (lit.)
mp
−172 °C (lit.)
density
0.362 g/mL at 20 °C (lit.)
SMILES string
CC
InChI
1S/C2H6/c1-2/h1-2H3
InChI key
OTMSDBZUPAUEDD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- High-pressure oxidation of ethane: The paper discusses the oxidation properties of ethane under high pressure, providing insights crucial for developing combustion models and understanding ethane′s behavior in various industrial processes (H Hashemi, JG Jacobsen, CT Rasmussen, 2017).
- Progress and prospects in catalytic ethane aromatization: This review highlights the advancements in converting ethane to more valuable aromatic hydrocarbons, showcasing the potential of ethane as a petrochemical feedstock (Y Xiang, H Wang, J Cheng, J Matsubu, 2018).
Packaging
Supplied in a carbon steel lecture bottle with a CGA180M/CGA110F needle valve installed.
Compatible with the following:
Compatible with the following:
- Aldrich® lecture-bottle station systems
- Aldrich® lecture-bottle gas regulators
Other Notes
See Technical Information Bulletin AL-151 Gas Regulators: Selection, Installation, and Operation
Legal Information
Aldrich is a registered trademark of Sigma-Aldrich Co. LLC
also commonly purchased with this product
Product No.
Description
Pricing
control valve
hose barb
Product No.
Description
Pricing
purge valve
Product No.
Description
Pricing
recommended
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Gas 1A - Press. Gas Liquefied gas
Storage Class Code
2A - Gases
WGK
nwg
Flash Point(F)
-211.0 °F - closed cup
Flash Point(C)
-135 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Isobel J Simpson et al.
Nature, 488(7412), 490-494 (2012-08-24)
After methane, ethane is the most abundant hydrocarbon in the remote atmosphere. It is a precursor to tropospheric ozone and it influences the atmosphere's oxidative capacity through its reaction with the hydroxyl radical, ethane's primary atmospheric sink. Here we present
Margaret A Greene et al.
Organic letters, 14(16), 4293-4296 (2012-05-10)
Stereospecific nickel-catalyzed cross-coupling reactions of benzylic 2-methoxyethyl ethers are reported for the preparation of enantioenriched 1,1-diarylethanes. The 2-methoxyethyl ether serves as a traceless directing group that accelerates cross-coupling. Chelation of magnesium ions is proposed to activate the benzylic C-O bond
Dario Frascari et al.
Bioresource technology, 128, 479-486 (2012-12-04)
A novel aerobic/anaerobic/aerobic treatment was implemented in batch reactors containing aquifer materials from a site contaminated by tetrachloroethylene (PCE), trichloroethylene (TCE), vinyl chloride (VC), 1,1,2-trichloroethane (1,1,2-TCA) and chloroform (CF). Consortia grown aerobically on methane, propane, n-pentane and n-hexane completely biodegraded
Reika Kanya et al.
The Journal of chemical physics, 136(20), 204309-204309 (2012-06-07)
Two-body Coulomb explosion processes of ethane (CH(3)CH(3)) and its isotopomers (CD(3)CD(3) and CH(3)CD(3)) induced by an intense laser field (800 nm, 1.0 × 10(14) W/cm(2)) with three different pulse durations (40 fs, 80 fs, and 120 fs) are investigated by
Calvin C H Chan et al.
Environmental science & technology, 46(18), 10154-10160 (2012-08-21)
Compound specific isotope analysis (CSIA) has been applied to monitor bioremediation of groundwater contaminants and provide insight into mechanisms of transformation of chlorinated ethanes. To date there is little information on its applicability for chlorinated methanes. Moreover, published enrichment factors
Protocols
Separation of Methane; Acetylene; Carbon monoxide; Water; Nitrogen; Carbon dioxide; Ethane; Ethylene
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service