47595
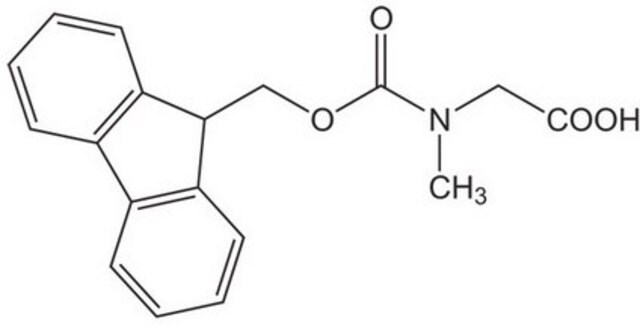
Fmoc-Sar-OH
≥98.0%
Synonym(s):
Fmoc-N-methylglycine, Fmoc-sarcosine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C18H17NO4
CAS Number:
Molecular Weight:
311.33
Beilstein:
6660958
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Quality Level
Assay
≥98.0%
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
application(s)
peptide synthesis
functional group
Fmoc
storage temp.
2-8°C
SMILES string
CN(CC(O)=O)C(=O)OCC1c2ccccc2-c3ccccc13
InChI
1S/C18H17NO4/c1-19(10-17(20)21)18(22)23-11-16-14-8-4-2-6-12(14)13-7-3-5-9-15(13)16/h2-9,16H,10-11H2,1H3,(H,20,21)
InChI key
ZHKQIADIIYMFOZ-UHFFFAOYSA-N
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tatsuhiko Kondo et al.
Plant & cell physiology, 52(1), 30-36 (2010-10-12)
CLAVATA3 (CLV3) is a plant peptide hormone in which the proline residues are post-translationally hydroxylated and glycosylated. CLV3 plays a key role in controlling the stem cell mass in the shoot meristem of Arabidopsis thaliana. In a previous report, we
Ewa Radzikowska et al.
Organic & biomolecular chemistry, 13(1), 269-276 (2014-11-05)
Chimeric oligonucleotides containing phosphodiester and phosphorothioate linkages have been obtained using the solid phase synthesis. The oligonucleotide parts possessing natural internucleotide phosphate bonds were assembled using commercially available nucleoside 3'-O-(2-cyanoethyl-N,N-diisopropylamino)phosphoramidites 7 whereas the phosphorothioate segment was built using nucleoside 3'-O-(2-thio-1,3,2-oxathiaphospholanes)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service