All Photos(1)
About This Item
Linear Formula:
C6(C6H5)6
CAS Number:
Molecular Weight:
534.69
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
>300 °C (lit.)
functional group
phenyl
SMILES string
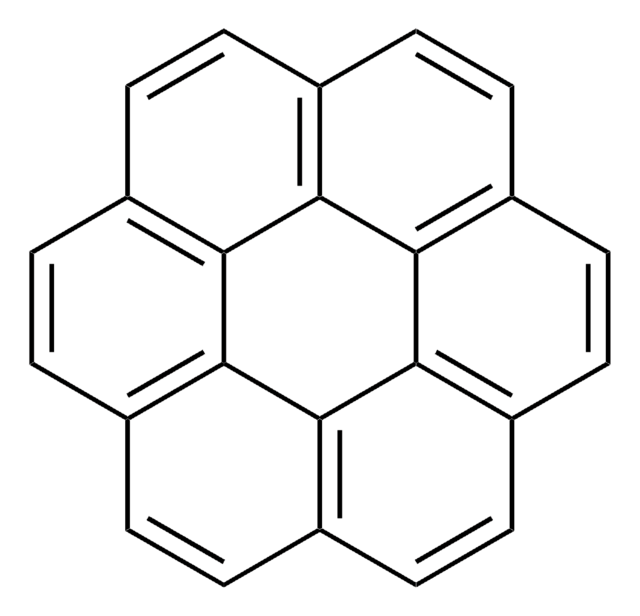
c1ccc(cc1)-c2c(-c3ccccc3)c(-c4ccccc4)c(-c5ccccc5)c(-c6ccccc6)c2-c7ccccc7
InChI
1S/C42H30/c1-7-19-31(20-8-1)37-38(32-21-9-2-10-22-32)40(34-25-13-4-14-26-34)42(36-29-17-6-18-30-36)41(35-27-15-5-16-28-35)39(37)33-23-11-3-12-24-33/h1-30H
InChI key
QBHWPVJPWQGYDS-UHFFFAOYSA-N
Application
Hexaphenylbenzene can be used as a starting material to synthesize:
- 1,2,3,4,5,6-Hexacyclohexylcyclohexane by Pd/C catalyzed hydrogenation reaction.
- Stable hexatrityl cations and porous organic polymers for applications in catalysis and gas storage.
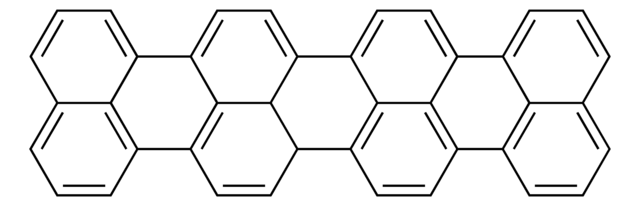
- Hexa-peri-hexabenzocoronene via one-pot substitution and oxidative cyclodehydrogenation reaction in the presence of t-BuCl/FeCl3.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A Practical One-Pot Synthesis of Soluble Hexa-peri-hexabenzocoronene and Isolation of Its Cation-Radical Salt
Rathore R and Burns CL
J. Org. React., 68(10), 4071-4074 (2003)
Yan-Fang Jing et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 238, 118443-118443 (2020-05-14)
A series of fluorescent coordination polymers (CPs) {[Cd2(CH3-bpeb)2(BDC)2] CP1, (BDC)0.5/(NH2-BDC)0.5-CP1, (BDC)0.34/(NH2-BDC)0.66-CP1, (BDC)0.25/(NH2-BDC)0.75-CP1, (BDC)0.2/(NH2-BDC)0.8-CP1, (NH2-BDC)-CP1} were prepared from conjugated ligand 4,4'-((2-methyl-1,4-phenylene)bis(ethene-2,1-diyl))bipyridine (CH3-bpeb), terephthalic acid (BDC), aminoterephthalic acid (NH2-BDC) and CdSO4 under solvothermal conditions. The fluorescence of aqueous suspensions of these CPs
From Hexaphenylbenzene to 1,2,3,4,5,6-Hexacyclohexylcyclohexane
Dillenburger M, et al.
Journal of the American Chemical Society, 142(30), 12916-12920 (2020)
Porous organic polymers based on propeller-like hexaphenylbenzene building units
Chen Q, et al.
Macromolecules, 44(14), 5573-5577 (2011)
Synthesis and isolation of polytrityl cations by utilizing hexaphenylbenzene and tetraphenylmethane scaffolds
Rathore R, et al.
The Journal of Organic Chemistry, 69(5), 1524-1530 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service







![[Bis(trifluoroacetoxy)iodo]benzene 97%](/deepweb/assets/sigmaaldrich/product/structures/238/293/71fcde9a-4afb-4cf5-9c22-8d8d68bf1ba4/640/71fcde9a-4afb-4cf5-9c22-8d8d68bf1ba4.png)
