All Photos(2)
About This Item
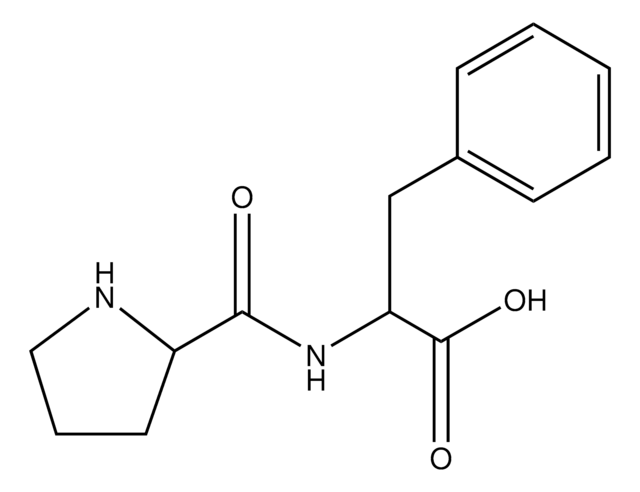
Empirical Formula (Hill Notation):
C14H18N2O3
CAS Number:
Molecular Weight:
262.30
MDL number:
UNSPSC Code:
12352202
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Assay
≥98% (TLC)
Quality Level
form
powder
color
white
storage temp.
−20°C
SMILES string
O=C(N1CCC[C@H]1C(O)=O)[C@@H](N)CC2=CC=CC=C2
InChI
1S/C14H18N2O3/c15-11(9-10-5-2-1-3-6-10)13(17)16-8-4-7-12(16)14(18)19/h1-3,5-6,11-12H,4,7-9,15H2,(H,18,19)
InChI key
WEQJQNWXCSUVMA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Oliver Trapp et al.
Electrophoresis, 25(2), 318-323 (2004-01-27)
Dynamic capillary electrophoresis (DCE) and computer simulation of the elution profiles with the stochastic model has been applied to determine the isomerization barriers of the angiotensin converting enzyme inhibitor enalaprilat. The separation of the rotational cis-trans isomeric drug has been
Enrique Martínez-Carranza et al.
Extremophiles : life under extreme conditions, 22(1), 73-85 (2017-11-13)
The Cuatro Ciénegas Basin (CCB) within the Chihuahuan Desert in México is an extremely oligotrophic oasis with negligible phosphorous levels, described as a hot spot of biodiversity, not only in stromatolites and microbial mats, but also in living forms in
R Gurunath et al.
Biochemical and biophysical research communications, 202(1), 241-245 (1994-07-15)
Non protein amino acids with strong secondary structure preferences are potentially useful in peptide design. alpha-Aminoisobutyric acid (Aib) is a powerful 'stereochemical director' of polypeptide chain folding, stabilizing helical conformations in diverse oligopeptide sequences. In an approach to the de
Joseph A Davis et al.
Indian journal of pharmacology, 44(6), 759-764 (2012-12-19)
Dipeptidyl peptidase IV (DPP-IV) inhibition to modulate the incretin effect is a proven strategy to treat type 2 diabetes mellitus. The present study describes the pharmacological profile of a novel DPP-IV inhibitor RBx-0128, as an antidiabetic agent. DPP-IV assay was
G Schoetz et al.
Electrophoresis, 22(12), 2409-2415 (2001-08-25)
Dynamic capillary electrophoresis (DCE) and computer simulation of the elution profiles with the theoretical plate and the stochastic model has been applied to determine the isomerization barriers of the three dipeptides L-alanyl-L-proline, L-leucyl-L-proline, and L-phenylalanyl-L-proline. The separation of the rotational
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service