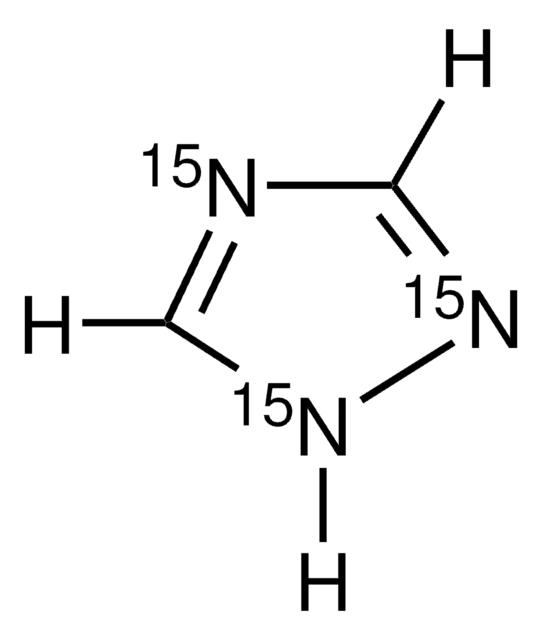
T46108
1,2,4-Triazole
98%
Synonym(s):
3,4-Diazapyrrole, 4H-1,2,4-Triazole, s-Triazole (8CI)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C2H3N3
CAS Number:
Molecular Weight:
69.07
Beilstein:
104767
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
bp
260 °C (lit.)
mp
119-121 °C (lit.)
SMILES string
c1nc[nH]n1
InChI
1S/C2H3N3/c1-3-2-5-4-1/h1-2H,(H,3,4,5)
InChI key
NSPMIYGKQJPBQR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
1,2,4-triazole and its derivatives are important structural moieties of many pharmaceutical drugs. Triazoles can also act as ligands to form coordination complexes with transition metal ions. Due to their electron-deficient nature, they exhibit excellent electron-transport and hole-blocking properties, making them promising organic materials in material science applications.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Repr. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 2
Flash Point(F)
338.0 °F
Flash Point(C)
170 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mononuclear, oligonuclear and polynuclear metal coordination compounds with 1, 2, 4-triazole derivatives as ligands.
Haasnoot JG
Coordination Chemistry Reviews, 200, 131-185 (2000)
Applications of Metal-Free 1, 2, 4-Triazole Derivatives in Materials Science.
Diaz-Ortiz A
Current Organic Chemistry, 19(7), 568-584 (2015)
1, 2, 4-Triazoles: Synthetic approaches and pharmacological importance.
Al-Masoudi IA
Chemistry of Heterocyclic Compounds, 42(11), 1377-1403 (2006)
Tomasz Plech et al.
European journal of medicinal chemistry, 60, 208-215 (2013-01-08)
Designed and synthesized 4-alkyl-1,2,4-triazole-3-thione derivatives showed significant anticonvulsant activity, determined in the maximal electroshock-induced seizure (MES) test. The chemical structure of all new compounds was confirmed by spectral methods ((1)H NMR, (13)C NMR, IR, MS). A sensitive and selective method
Christopher R Murdock et al.
Inorganic chemistry, 52(4), 2182-2187 (2013-02-07)
A semirigid di-1,2,4-triazole ligand leads to formation of the MOF [Cu(2)(L)(2)(SO(4))(Br)(2)]·xH(2)O (1). The framework structure of 1 flexes reversibly upon removal or addition of water to form semihydrated ([Cu(2)(L)(2)(SO(4))(Br)(2)]·4H(2)O) and dehydrated ([Cu(2)(L)(2)(SO(4))(Br)(2)]·0H(2)O) MOFs, 1' and 1″, respectively. Single-crystal X-ray analysis
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service