761885
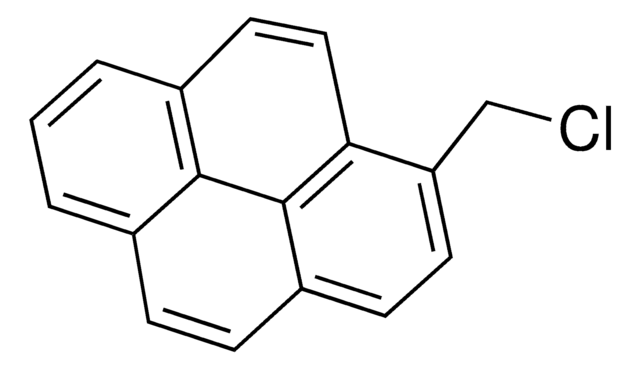
1-(Bromomethyl)pyrene
Synonym(s):
1-Pyrenylmethyl bromide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C17H11Br
CAS Number:
Molecular Weight:
295.17
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
solid
mp
140-145 °C
functional group
bromo
SMILES string
BrCc1ccc2ccc3cccc4ccc1c2c34
InChI
1S/C17H11Br/c18-10-14-7-6-13-5-4-11-2-1-3-12-8-9-15(14)17(13)16(11)12/h1-9H,10H2
InChI key
UGMXRPVWWWDPFC-UHFFFAOYSA-N
Application
1-(Bromomethyl)pyrene is widely used in the synthesis of fluorophores for the fluorescent sensing of variety of analytes including metal ions like Cd2+, Zn2+ and adenosine triphosphate (ATP) sensing at physiological pH. It is also used in the synthesis of photoinitiators (PIs) for the radical polymerization of acrylates and the cationic polymerization of epoxy-silicone and vinyl ethers.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A pyrenyl-appended triazole-based calix [4] arene as a fluorescent sensor for Cd2+ and Zn2+.
Park S Y, et al.
The Journal of Organic Chemistry, 73(21), 8212-8218 (2008)
A tong-like fluorescence sensor for metal ions: perfect conformational switch of hinge sugar by pyrene stacking.
Yuasa H, et al.
Organic & Biomolecular Chemistry, 2(24), 3548-3556 (2004)
New insights into radical and cationic polymerizations upon visible light exposure: role of novel photoinitiator systems based on the pyrene chromophore.
Tehfe M A, et al.
Polym. Chem., 4(5), 1625-1634 (2013)
Unique sandwich stacking of pyrene-adenine-pyrene for selective and ratiometric fluorescent sensing of ATP at physiological pH.
Xu Z, et al.
Journal of the American Chemical Society, 131(42), 15528-15533 (2009)
Jing Wang et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 228, 117725-117725 (2019-11-14)
We developed PIM, a pyrene-based fluorescence sensor bearing an imidazole moiety and a carbonyl group as the binding sites for Fe3+ ions. The pyrene-based control compounds 1 and 2 were synthesized to demonstrate the structure-activity relationships. Compound 1, which contained
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service