427691
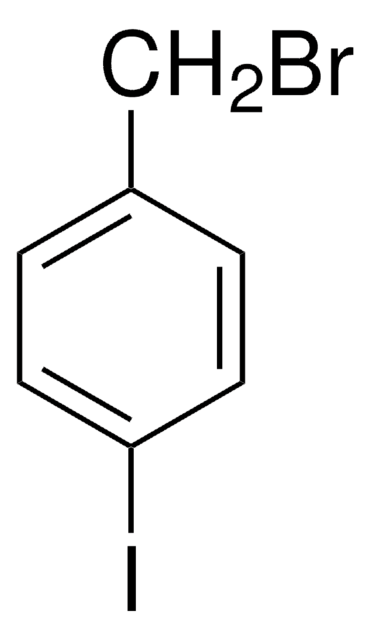
3-Iodobenzyl bromide
95%
Synonym(s):
α-Bromo-m-iodotoluene, 1-(Bromomethyl)-3-iodobenzene, 3-(Bromomethyl)iodobenzene, m-Iodobenzylbromide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
IC6H4CH2Br
CAS Number:
Molecular Weight:
296.93
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
mp
46-51 °C (lit.)
functional group
bromo
iodo
SMILES string
BrCc1cccc(I)c1
InChI
1S/C7H6BrI/c8-5-6-2-1-3-7(9)4-6/h1-4H,5H2
InChI key
BACZSVQZBSCWIG-UHFFFAOYSA-N
Related Categories
General description
3-Iodobenzyl bromide (3-IBBr, m-iodobenzyl bromide) is a meta-isomer of iodobenzyl bromide. It can be synthesized by the bromination of m-iodotoluene.
Application
3-Iodobenzyl bromide (3-IBBr, m-iodobenzyl bromide) was used as a derivatization reagent for the extraction and purification of thiouracil (TU).
It may be used in the synthesis of the following:
It may be used in the synthesis of the following:
- meta-substituted phenylalanine derivatives
- N6-substituted aristeromycin derivative
- (N)-methanocarba-N6-(3-iodobenzyl)adenosine
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Phase Transfer Catalyst (PTC) Catalyzed Alkylations of Glycinamides for Asymmetric Syntheses of alpha-Amino Acid Derivatives.
Park YS, et al.
Bull. Korean Chem. Soc., 22(9), 958-962 (2001)
Reaction of some sulfonyl halides with trypsin.
Kuranova IP and Konareva NV.
Bulletin of the Academy of Sciences of the USSR, Division of chemical science, 22(10), 2251-2254 (1973)
Julie A L Kiebooms et al.
Applied and environmental microbiology, 80(23), 7433-7442 (2014-09-28)
In recent years, the frequent detection of the banned thyreostat thiouracil (TU) in livestock urine has been related to endogenous TU formation following digestion of glucosinolate-rich Brassicaceae crops. Recently, it was demonstrated that, upon in vitro digestion of Brassicaceae, fecal
K A Jacobson et al.
Journal of medicinal chemistry, 43(11), 2196-2203 (2000-06-08)
Adenosine receptor agonists have cardioprotective, cerebroprotective, and antiinflammatory properties. We report that a carbocyclic modification of the ribose moiety incorporating ring constraints is a general approach for the design of A(1) and A(3) receptor agonists having favorable pharmacodynamic properties. While
Barbara Woźniak et al.
Journal of veterinary research, 62(4), 511-517 (2019-02-08)
In the European Union, the use of thyreostatic drugs for fattening slaughter animals has been banned since 1981 under Council Directive 81/602/EEC. For protection of consumer health against unwanted residues and in compliance with Directive 96/23, each EU country must
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service