392847
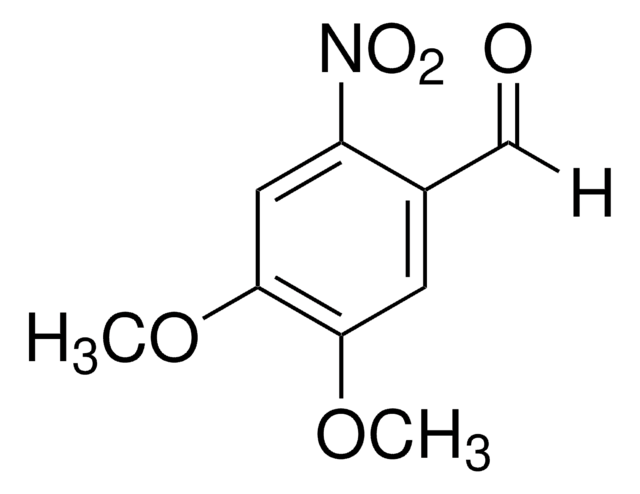
4,5-Dimethoxy-2-nitrobenzyl alcohol
98%
Synonym(s):
6-Nitroveratryl alcohol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
O2NC6H2(OCH3)2CH2OH
CAS Number:
Molecular Weight:
213.19
Beilstein:
1880093
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
145-148 °C (lit.)
SMILES string
COc1cc(CO)c(cc1OC)[N+]([O-])=O
InChI
1S/C9H11NO5/c1-14-8-3-6(5-11)7(10(12)13)4-9(8)15-2/h3-4,11H,5H2,1-2H3
InChI key
WBSCOJBVYHQOFB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4,5-Dimethoxy-2-nitrobenzyl alcohol (6-Nitroveratryl Alcohol) is 2-nitrobenzyl alcohol derivative. It has been reported to be one of the oxidation products of veratryl (3,4-dimethoxybenzyl) alcohol by lignin peroxidase (isolated from Phanerochaete chrysosporium).
Application
4,5-Dimethoxy-2-nitrobenzyl alcohol (6-nitroveratryl alcohol) is suitable reagent used in the synthesis of 4,5-dimethoxy-2-nitrobenzyl methacrylate, a photolabile monomer and 2-(4-((4-(4,5-dimethoxy-2-nitrobenzyloxy)phenyl)cyclohexylidene)methyl)phenoxy)-N,N-dimethylethanamine, a caged cyclofen-OH ligand.
It may be used in the synthesis of the following:
It may be used in the synthesis of the following:
- 1-[[(chlorocarbonyl)oxy]methyl]-4,5-dimethoxy-2-nitrobenzene
- bis(4,5-dimethoxy-2-nitrophenyl)ethylene glycol, a photolabile protecting group
- optically-sensitive monomer
- nitroveratryl (NV) protected α-hydroxyacetic acid (αG) (NV-αG-OH), required in the preparation of nitroveratryl (NV) protected cyanomethyl (CM) ester of α-hydroxyacetic acid (αG) (NV-αG-CM)
- 4,5-dimethoxy-2-nitrobenzyl p-nitro-phenylcarbonate
- 6-nitroveratryloxycarbonyl chloride (NVOCCl), a reagent used in the protection of amino function in amino sugars
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Peptide backbone mutagenesis of putative gating hinges in a potassium ion channel.
Yasuo Nagaoka et al.
Chembiochem : a European journal of chemical biology, 9(11), 1725-1728 (2008-06-11)
Nadezda Fomina et al.
Journal of the American Chemical Society, 132(28), 9540-9542 (2010-06-24)
A new light-sensitive polymer containing multiple light-sensitive triggering groups along the backbone and incorporating a quinone-methide self-immolative moiety was developed and formulated into nanoparticles encapsulating a model pharmaceutical Nile Red. Triggered burst release of the payload upon irradiation and subsequent
Bis (4, 5-dimethoxy-2-nitrophenyl) ethylene glycol: a new and efficient photolabile protecting group for aldehydes and ketones.
Kantevari S, et al.
Tetrahedron, 61(24), 5849-5854 (2005)
Photosensitive protecting groups of amino sugars and their use in glycoside synthesis. 2-nitrobenzyloxycarbonylamino and 6-nitroveratryloxycarbonylamino derivatives.
Amit B, et al.
The Journal of Organic Chemistry, 39(2), 192-196 (1974)
Deepak Kumar Sinha et al.
Chembiochem : a European journal of chemical biology, 11(5), 653-663 (2010-02-27)
We have implemented a noninvasive optical method for the fast control of protein activity in a live zebrafish embryo. It relies on releasing a protein fused to a modified estrogen receptor ligand binding domain from its complex with cytoplasmic chaperones
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service