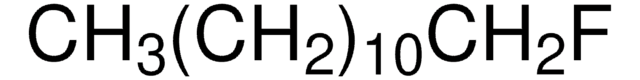All Photos(1)
About This Item
Linear Formula:
CH3(CH2)9I
CAS Number:
Molecular Weight:
268.18
Beilstein:
1735228
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
>5 (vs air)
vapor pressure
0.01 mmHg ( 20 °C)
Assay
98%
form
liquid
contains
copper as stabilizer
refractive index
n20/D 1.485 (lit.)
bp
132 °C/15 mmHg (lit.)
density
1.257 g/mL at 25 °C (lit.)
functional group
alkyl halide
iodo
SMILES string
CCCCCCCCCCI
InChI
1S/C10H21I/c1-2-3-4-5-6-7-8-9-10-11/h2-10H2,1H3
InChI key
SKIDNYUZJPMKFC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Electrochemical reduction of 1-iododecane at mercury cathodes in DMF containing tetra-n-butylammonium perchlorate or tetramethyl ammonium perchlorate (supporting electrolyte) has been investigated.
Application
1-Iododecane has been used in the preparation of 9-nonadecanone via palladium-catalyzed carbonylative cross-coupling reaction with 9-octyl-9-borabicyclo[3.3.l]nonane.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Electrochemical reduction of 1-iododecane and 1-bromodecane at a mercury cathode in dimethylformamide.
McNamee GM, et al.
Journal of the American Chemical Society, 99(6), 1831-1835 (1977)
Niluksha Walalawela et al.
Photochemistry and photobiology, 95(5), 1160-1168 (2019-03-19)
In order to develop a new long alkane chain pterin that leaves the pterin core largely unperturbed, we synthesized and photochemically characterized decyl pterin-6-carboxyl ester (CapC) that preserves the pterin amide group. CapC contains a decyl-chain at the carboxylic acid
Palladium-catalyzed carbonylative cross-coupling reaction of iodoalkanes with 9-alkyl-9-BBN derivatives. A direct and selective synthesis of ketones.
Ishiyama T and Miyaura N.
Tetrahedron Letters, 32(47), 6923-6926 (1991)
Minhuan Li et al.
Science advances, 6(27), eaaw8938-eaaw8938 (2020-07-14)
Most systems have more than two stable crystalline states in the phase diagram, which is known as polymorphism. Crystallization in such a system is often under strong influence of competing orderings linked to those crystals. However, how such competition affects
María José Sosa et al.
Photochemistry and photobiology, 97(1), 80-90 (2020-07-07)
Mono- and bis-decylated lumazines have been synthesized and characterized. Namely, mono-decyl chain [1-decylpteridine-2,4(1,3H)-dione] 6a and bis-decyl chain [1,3-didecylpteridine-2,4(1,3H)-dione] 7a conjugates were synthesized by nucleophilic substitution (SN 2) reactions of lumazine with 1-iododecane in N,N-dimethylformamide (DMF) solvent. Decyl chain coupling occurred
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service