157163
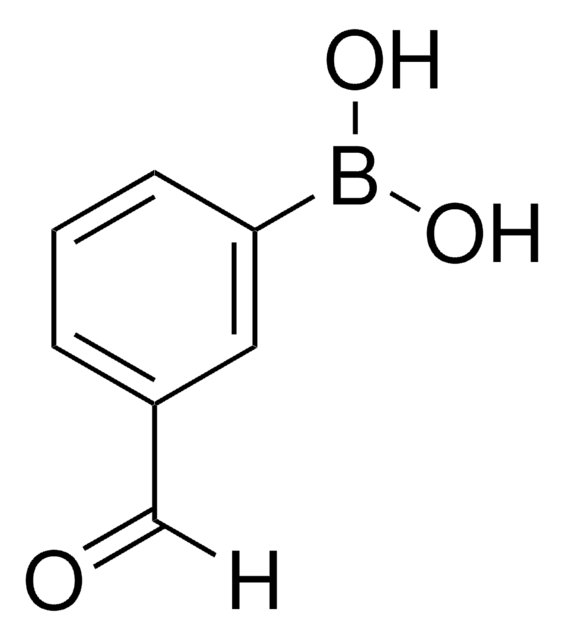
3-Cyanobenzoic acid
98%
Synonym(s):
Isophthalic acid mononitrile
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
NCC6H4CO2H
CAS Number:
Molecular Weight:
147.13
Beilstein:
1862566
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder
mp
220-224 °C (lit.)
functional group
carboxylic acid
nitrile
SMILES string
OC(=O)c1cccc(c1)C#N
InChI
1S/C8H5NO2/c9-5-6-2-1-3-7(4-6)8(10)11/h1-4H,(H,10,11)
InChI key
GYLKKXHEIIFTJH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
3-Cyanobenzoic acid was used in the preparation of new Co(II)-doped Zn(II)-tetrazole-benzoate coordination polymers via in situ [2+3] cycloaddition reactions with NaN3 in the presence of Zn(II) and/or Co(II) salts under hydrothermal conditions. It was also used in the synthesis of three-dimensional coordination polymer, [Mn3(OH)2Na2(3-cnba)6]n (3-Hcnba = 3-cyanobenzoic acid).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Wei-Chao Song et al.
Inorganic chemistry, 48(8), 3792-3799 (2009-03-24)
In our continuing efforts to explore the effects of ligand modifications on the structures and properties of their metal complexes, we studied the in situ [2 + 3] cycloaddition reactions of benzonitrile, o-phthalodinitrile, 3-cyanobenzoic acid, 4-cyanobenzoic acid with NaN(3) in
Jin-Tang Li et al.
Inorganic chemistry, 44(13), 4448-4450 (2005-06-21)
A three-dimensional coordination polymer, [Mn3(OH)2Na2(3-cnba)6]n (1) (3-Hcnba = 3-cyanobenzoic acid), has been synthesized by the reaction of MnCl2, NaN3, and 3-Hcnba in water. Its crystal structure was determined by single-crystal X-ray diffraction. Magnetic studies show that the complex behaves as
Tomoko Abe et al.
The Journal of antibiotics, 70(4), 435-442 (2016-10-13)
The adenylation domain of nonribosomal peptide synthetase (NRPS) is responsible for the selective substrate recognition and its activation (as an acyl-O-AMP intermediate) during ATP consumption. DhbE, a stand-alone adenylation domain, acts on an aromatic acid, 2,3-dihydroxybenzoic acid (DHB). This activation
Homan Kang et al.
Scientific reports, 5, 10144-10144 (2015-05-29)
Recently, preparation and screening of compound libraries remain one of the most challenging tasks in drug discovery, biomarker detection, and biomolecular profiling processes. So far, several distinct encoding/decoding methods such as chemical encoding, graphical encoding, and optical encoding have been
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service