Products may be shipped at a different temperature than the recommended long-term storage temperature. If the product quality is sensitive to short-term exposure to conditions other than the recommended long-term storage, it will be shipped on wet or dry-ice. If the product quality is NOT affected by short-term exposure to conditions other than the recommended long-term storage, it will be shipped at ambient temperature. As shipping routes are configured for minimum transit times, shipping at ambient temperature helps control shipping costs for our customers. For more information, please refer to the Storage and Transport Conditions document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/316/622/storage-transport-conditions-mk.pdf
V2002
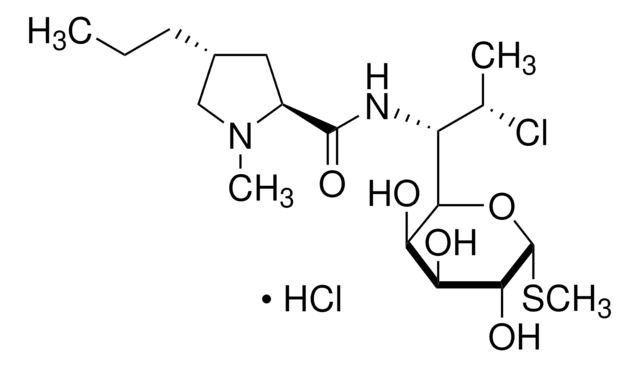
Vancomycin hydrochloride from Streptomyces orientalis
≥900 μg per mg (as vancomycin base)
Synonym(s):
Vancomycin, Vancomycin HCL
Select a Size
Select a Size
About This Item
Recommended Products
biological source
Streptomyces orientalis
Quality Level
form
powder
storage condition
(Keep container tightly closed in a dry and well-ventilated place.)
concentration
≥900 μg/mg (as vancomycin base)
color
, off-white to brown or White to orange-brown
antibiotic activity spectrum
Gram-positive bacteria
Mode of action
cell wall synthesis | interferes
storage temp.
2-8°C
SMILES string
Cl[H].CO[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@@H]1O[C@H]2C[C@](C)(N)[C@@H](O)[C@@H](C)O2.CN[C@H](CC(C)C)C(=O)NC3[C@H](O)c4ccc(Oc5cc6Oc7ccc(cc7Cl)[C@@H](O)[C@H]8NC(=O)[C@H](NC(=O)[C@H](NC(=O)[C@H](CC(N)=O)NC3=O)c(c5)c6)c9ccc(O)c(c9)-c%10c(O)cc(O)cc%10[C@@H](NC8=O)C(O)=O)c(Cl)c4
InChI
1S/C66H75Cl2N9O24.ClH/c1-23(2)12-34(71-5)58(88)76-49-51(83)26-7-10-38(32(67)14-26)97-40-16-28-17-41(55(40)101-65-56(54(86)53(85)42(22-78)99-65)100-44-21-66(4,70)57(87)24(3)96-44)98-39-11-8-27(15-33(39)68)52(84)50-63(93)75-48(64(94)95)31-18-29(79)19-37(81)45(31)30-13-25(6-9-36(30)80)46(60(90)77-50)74-61(91)47(28)73-59(89)35(20-43(69)82)72-62(49)92;/h6-11,13-19,23-24,34-35,42,44,46-54,56-57,65,71,78-81,83-87H,12,20-22,70H2,1-5H3,(H2,69,82)(H,72,92)(H,73,89)(H,74,91)(H,75,93)(H,76,88)(H,77,90)(H,94,95);1H/t24-,34+,35-,42+,44-,46+,47+,48-,49+,50-,51+,52+,53+,54-,56+,57+,65-,66-;/m0./s1
InChI key
LCTORFDMHNKUSG-XTTLPDOESA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- in the research the effects of antibiotic-induced depletion of Firmicutes and Bacteroidetes on dysregulation of energy homeostasis in obesity[1]
- in the research of non-O157 Shiga toxin-producing Escherichia coli Isolates from Bovine Farms[2]
- to research the antimicrobial susceptibility of Bifidobacterium strains in various organisms[3]
Biochem/physiol Actions
Antimicrobial Spectrum: Active against Gram-positive bacteria
Features and Benefits
- Effective against a wide range of Gram-positive bacteria, including MRSA
- Commonly used in Cell Biology and Biochemical applications
Packaging
Other Notes
comparable product
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
β-lactam antibacterials inhibit transpeptidase enzymes, preventing peptidoglycan assembly in both Gram-positive and Gram-negative bacteria.
-
How is shipping temperature determined? And how is it related to the product storage temperature?
1 answer-
Helpful?
-
-
How can I determine the shelf life / expiration / retest date of this product?
1 answer-
If this product has an expiration or retest date, it will be shown on the Certificate of Analysis (COA, CofA). If there is no retest or expiration date listed on the product's COA, we do not have suitable stability data to determine a shelf life. For these products, the only date on the COA will be the release date; a retest, expiration, or use-by-date will not be displayed.
For all products, we recommend handling per defined conditions as printed in our product literature and website product descriptions. We recommend that products should be routinely inspected by customers to ensure they perform as expected.
For products without retest or expiration dates, our standard warranty of 1 year from the date of shipment is applicable.
For more information, please refer to the Product Dating Information document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/449/386/product-dating-information-mk.pdfHelpful?
-
-
What is the Department of Transportation shipping information for this product?
1 answer-
Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
Helpful?
-
-
What is Product V2002, Vancomycin, soluble in?
1 answer-
This product is soluble in water (50 mg/ml), forming a clear, colorless to faint yellow solution. It is also moderately soluble in methanol, but insoluble in the higher alcohols, acetone, and ether. Low concentrations of urea increase the solubility in neutral aqueous solutions, and ammonium sulfate and sodium chloride precipitate the antibiotic from acidic solutions.
Helpful?
-
-
What is the retest period for Product V2002, Vancomycin?
1 answer-
At this time, Product No. V2002 is assigned a retest period of 2 years from the quality control release date. This interval may change if we are able to collect sufficient data from future retesting.
Helpful?
-
-
How long are Product V2002, Vancomycin, solutions stable for?
1 answer-
Solutions of vancomycin in physiological solutions such as 0.9% saline and 5% glucose are stable for 17 days at 24 °C and 63 days and 5 °C and -10 °C.
Helpful?
-
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service