SBR00047
Rhodamine B labeled Ramoplanin
For fluorescent microbial imaging
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C146H184ClN23O46S2
Molecular Weight:
3096.73
UNSPSC Code:
12352116
NACRES:
NA.76
Recommended Products
General description
Rhodamine B labeled Ramoplanin is a fluorescent derivative of Ramoplanin, with a similar mode of action as Ramoplanin. It allows fluorescent labeling of Gram-positive bacteria and their detection by common fluorescent instruments. Ramoplanin is a lipoglycodepsipeptide antibiotic that is produced by the fermentation of Actinoplanes species. Fluorescent antibiotics are obtained by a synthetic conjugation of an antibiotic to a fluorophore.
Application
Fluorescent antibiotics can be used for many applications including:
- Antimicrobial resistance research
- Bacterial visualization and imaging
- Parent antibiotic mode of action research and new antibiotic discovery
- Toxicity studies
- Diagnosis of bacterial infections and tracking their uptake in vivo
Biochem/physiol Actions
Ramoplanin blocks the bacterial cell wall biosynthesis by specifically binding to the first sugar of lipid I. N-Acetylmuramic acid thus interfering with peptidoglycan production.
Mode of Action: Is different from the d-Ala-d-Ala that are targeted by vancomycin and shows no cross-resistance with other glycopeptides.
Activity Spectrum: Effective against a broad-spectrum activity against Gram-positive pathogens both in vitro and in vivo including Enterococci, Staphylococci, Bacilli, Streptococci, Listeria monocytogenes, and Gram-positive anaerobes such as Clostridrium difficile. However, Rhodamine B labeled Ramoplanin presents a MIC decrease in the magnitude of two to five orders in bacterial activity in comparison to the parent Ramoplanin.
Mode of Action: Is different from the d-Ala-d-Ala that are targeted by vancomycin and shows no cross-resistance with other glycopeptides.
Activity Spectrum: Effective against a broad-spectrum activity against Gram-positive pathogens both in vitro and in vivo including Enterococci, Staphylococci, Bacilli, Streptococci, Listeria monocytogenes, and Gram-positive anaerobes such as Clostridrium difficile. However, Rhodamine B labeled Ramoplanin presents a MIC decrease in the magnitude of two to five orders in bacterial activity in comparison to the parent Ramoplanin.
Features and Benefits
High quality antibiotic suitable for mulitple research applications
Analysis Note
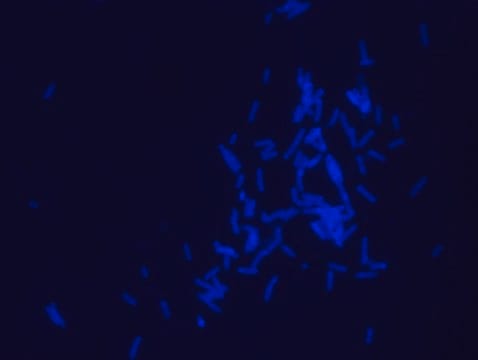
- Fluorescent microscopy application: Rhodamine B labeled Ramoplanin has excitation/emission wavelength range at 550-560 nm/590-600 nm.
- It is recommended to dissolve the Rhodamine B labeled Ramoplanin in DMSO to a concentration of 2 mg/mL.
- The recommended working concentration in fluorescent microscopy imaging application is 10μM. The mentioned concentration was used for Bacillus subtills staining (see image).
- Aliquots of the DMSO solution can be stored at −20 °C, protected from light for at least one month.
Other Notes
For additional information on our range of Biochemicals, please complete this form.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Predrag Cudic et al.
Proceedings of the National Academy of Sciences of the United States of America, 99(11), 7384-7389 (2002-05-29)
The peptide antibiotic ramoplanin inhibits bacterial peptidoglycan (PG) biosynthesis by interrupting late-stage membrane-associated glycosyltransferase reactions catalyzed by the transglycosylase and MurG enzymes. The mechanism of ramoplanin involves sequestration of lipid-anchored PG biosynthesis intermediates, physically occluding these substrates from proper utilization
Debra K Farver et al.
The Annals of pharmacotherapy, 39(5), 863-868 (2005-03-24)
To review the pharmacology, antimicrobial activity, pharmacokinetics, clinical applications, and safety of ramoplanin, a lipoglycodepsipeptide antibiotic. Information was obtained from MEDLINE and BIOSIS databases (1984-August 2004) and Oscient Pharmaceuticals using the key words ramoplanin, A 16686, A 16686A, and MDL
Xiao Fang et al.
Molecular bioSystems, 2(1), 69-76 (2006-08-02)
The lipoglycodepsipeptide antibiotic ramoplanin is proposed to inhibit bacterial cell wall biosynthesis by binding to intermediates along the pathway to mature peptidoglycan, which interferes with further enzymatic processing. Two sequential enzymatic steps can be blocked by ramoplanin, but there is
Kittichoat Tiyanont et al.
Proceedings of the National Academy of Sciences of the United States of America, 103(29), 11033-11038 (2006-07-13)
The peptidoglycan (PG) layers surrounding bacterial cells play an important role in determining cell shape. The machinery controlling when and where new PG is made is not understood, but is proposed to involve interactions between bacterial actin homologs such as
M Rhia L Stone et al.
Trends in biotechnology, 36(5), 523-536 (2018-02-27)
Better understanding how multidrug-resistant (MDR) bacteria can evade current and novel antibiotics requires a better understanding of the chemical biology of antibiotic action. This necessitates using new tools and techniques to advance our knowledge of bacterial responses to antibiotics, ideally
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service