06655
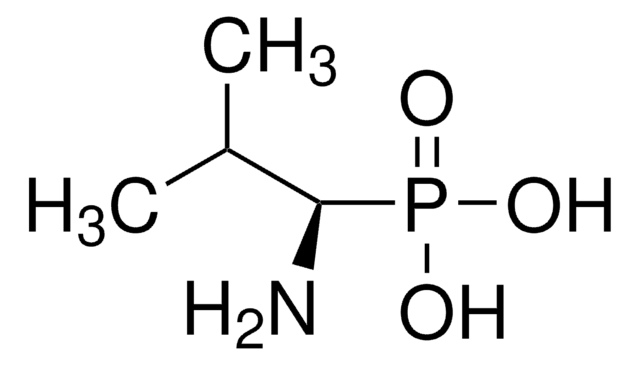
(R)-(−)-1-Aminoethylphosphonic acid
≥97.0% (NT)
Synonym(s):
L-(−)-1-Aminoethylphosphonic acid, L-Ala(P)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3CH(NH2)P(O)(OH)2
CAS Number:
Molecular Weight:
125.06
Beilstein:
4291032
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥97.0% (NT)
optical activity
[α]20/D −4.8±0.5°, c = 5% in H2O
storage temp.
2-8°C
SMILES string
C[C@H](N)P(O)(O)=O
InChI
1S/C2H8NO3P/c1-2(3)7(4,5)6/h2H,3H2,1H3,(H2,4,5,6)/t2-/m1/s1
InChI key
UIQSKEDQPSEGAU-UWTATZPHSA-N
Looking for similar products? Visit Product Comparison Guide
General description
(R)-(-)-1-Aminoethylphosphonic acid is a synthetic analog of L-alanine that shows antibacterial property.
Application
(R)-(−)-1-Aminoethylphosphonic acid can be used to prepare copper(II) heteroligand complexes, which are employed in the solution equilibrium studies.
Other Notes
Di- to tetrapeptides with this amino acid analogue at the "P-terminal" are bactericides and synergistic potentiators of penicillins and cephalophorins.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Reaction of Alanine Racemase with 1-Aminoethylphosphonic Acid Forms a Stable External Aldimine
Stamper CGF, et al.
Biochemistry, 37(29), 10438-10445 (1998)
F R Atherton et al.
Antimicrobial agents and chemotherapy, 15(5), 677-683 (1979-05-01)
Peptide mimetics with C-terminal residues simulating natural amino acids have been designed as inhibitors of bacterial cell wall biosynthesis. The phosphonopeptide series consisting of various l and d residues of natural amino acids combined with 1-aminoalkyl (and aryl-alkyl-) phosphonic acid
J G Allen et al.
Antimicrobial agents and chemotherapy, 16(3), 306-313 (1979-09-01)
The metabolism and pharmacokinetics of a synthetic antibacterial phosphonodipeptide, alafosfalin, have been studied in rats, baboons, and human volunteers. The compound was rapidly absorbed from the injection site after subcutaneous and intramuscular administration and gave peak plasma concentrations at 15
Stabilities and coordination modes of α-alaninephosphonic acid in copper (II) heteroligand complexes with ethylenediamine, diethylenetriamine or N, N, N′, N′, N″-pentamethyldiethylene triamine in aqueous solution
Kamecka A, et al.
Journal of Solution Chemistry, 40(6), 1041-1054 (2011)
Phosphonopeptides, a new class of synthetic antibacterial agents.
J G Allen et al.
Nature, 272(5648), 56-58 (1978-03-02)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service