All Photos(1)
About This Item
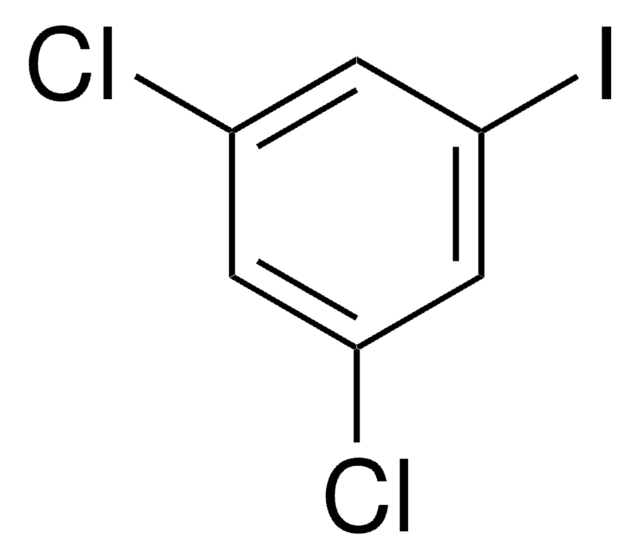
Linear Formula:
IC6H2(OCH3)2CHO
CAS Number:
Molecular Weight:
292.07
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
69-72 °C (lit.)
functional group
aldehyde
iodo
SMILES string
COc1cc(C=O)cc(I)c1OC
InChI
1S/C9H9IO3/c1-12-8-4-6(5-11)3-7(10)9(8)13-2/h3-5H,1-2H3
InChI key
MVPNBXPAUYYZAF-UHFFFAOYSA-N
General description
3-Iodo-4,5-dimethoxybenzaldehyde is an aryl iodide having an aldehyde group. It can be synthesized starting from 3,4-dihydroxy-5-iodobenzaldehyde.
Application
3-Iodo-4,5-dimethoxybenzaldehyde was used in the preparation of 3-iodo-4,4′,5-trimethoxy-3′-O-tert-butyldiphenylsilyl-Z-stilbene and 3-iodo-4,4′,5-trimethoxy-3′-O-tert-butyldiphenylsilyl-E-stilbene. It may be used for the synthesis of chloropeptin I and chloropeptin II.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
George R Pettit et al.
Journal of natural products, 75(3), 385-393 (2012-02-14)
Toward the objective of designing a structurally modified analogue of the combretastatin A-4 phosphate prodrug (1b) with the potential for increased specificity toward thyroid carcinoma, synthesis of a series of iodocombstatin phosphate (11a-h) and diiodocombstatin phosphate prodrugs (12a-h) has been
Joie Garfunkle et al.
Journal of the American Chemical Society, 131(44), 16036-16038 (2009-10-21)
The first total synthesis of chloropeptin II (1, complestatin) is disclosed. Key elements of the approach include the use of an intramolecular Larock indole synthesis for the initial macrocyclization, adopting conditions that permit utilization of a 2-bromoaniline, incorporating a terminal
Approaches to iodinated derivatives of vanillin and isovanillin.
Nammalwar B, et al.
Organic preparations and procedures international, 44(2), 146-146 (2012)
Nucleophilic catalysis of the iodine-zinc exchange reaction: preparation of highly functionalized diaryl zinc compounds.
Florian F Kneisel et al.
Angewandte Chemie (International ed. in English), 43(8), 1017-1021 (2004-02-18)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![2-[(Trimethylsilyl)ethynyl]benzaldehyde 97%](/deepweb/assets/sigmaaldrich/product/structures/977/954/5bc6c647-e9d2-4bf6-bf94-6ac20e3f476e/640/5bc6c647-e9d2-4bf6-bf94-6ac20e3f476e.png)