All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H9N3O3
CAS Number:
Molecular Weight:
171.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
135-137 °C (lit.)
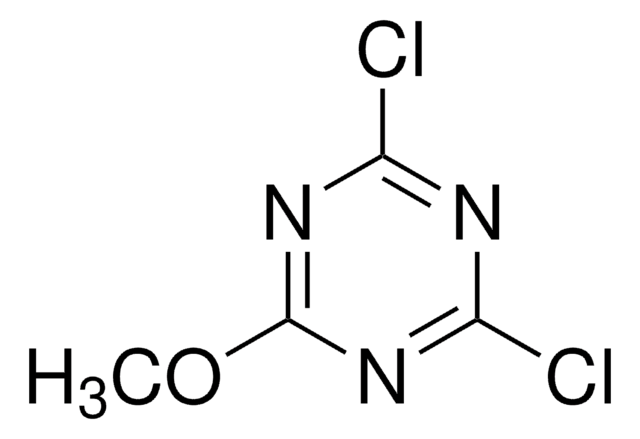
SMILES string
COc1nc(OC)nc(OC)n1
InChI
1S/C6H9N3O3/c1-10-4-7-5(11-2)9-6(8-4)12-3/h1-3H3
InChI key
DFUGJTBMQKRCPI-UHFFFAOYSA-N
General description
2,4,6-Trimethoxy-1,3,5-triazine (TMT) is a triazine derivative. It exists in three different polymorphic forms: α-, β- and γ-. 2,4,6-Trimethoxy-1,3,5-triazine molecules are planar in all the three polymorphs. Polymorphic forms differ in the mode of packing of the molecules in the crystal. Kinetics and mechanism of the reaction of radiolytically produced hydrated electron with TMT using pulse and steady-state radiolysis techniques has been studied. TMT undergoes methyl transfer in the melt and in the solid-state, to afford, 2,4,6-trioxo-1,3,5-trimethylazine.
Application
2,4,6-Trimethoxy-1,3,5-triazine was used to investigate the molecularly imprinted solid phase extraction (MISPE) for the isolation of melamine (ML) in food products.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Rani Varghese et al.
Journal of agricultural and food chemistry, 54(21), 8171-8176 (2006-10-13)
A study is made of the kinetics and mechanism of the reaction of radiolytically produced hydrated electron (e-(aq)) with some triazine derivatives [1,3,5-triazine (T), 2,4,6-trimethoxy-1,3,5-triazine (TMT), 2,4-dioxohexahydro-1,3,5-triazine (DHT), 6-chloro N-ethyl N-(1-methylethyl)-1,3,5-triazine 2,4-diamine (atrazine, AT), and cyanuric acid (CA)] in aqueous
Natalya Fridman et al.
Acta crystallographica. Section B, Structural science, 60(Pt 1), 97-102 (2004-01-22)
2,4,6-trimethoxy-1,3,5-triazine was found to exhibit three different polymorphs. The alpha-polymorph undergoes reversible phase transformation to the beta-polymorph at 340 K with an enthalpy of 3.9 kJ mol(-1). The heat of fusion of the beta-polymorph at 404 K is 18.1 kJ
Thermal rearrangement of cyanurates in the solid state.
Kaftory M and Handelsman-Benory E.
Mol. Cryst. Liq. Cryst., 240(1), 241-249 (1994)
Manuela Curcio et al.
Journal of agricultural and food chemistry, 58(22), 11883-11887 (2010-10-26)
A molecularly imprinted polymer able to recognize melamine in partially aqueous medium was synthesized using methacrylic acid as functional monomer and ethylene glycol dimethacrylate as cross-linking agent. The bound specificity and selectivity of the obtained material were verified by performing
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service