380768
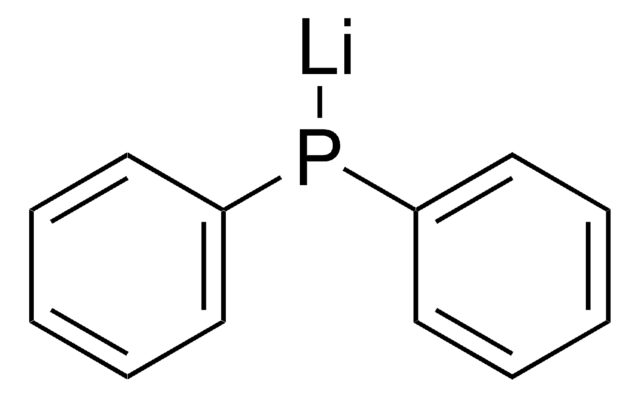
Potassium diphenylphosphide solution
0.5 M in THF
Synonym(s):
Potassium diphenylphosphine, Diphenylphosphine potassium salt, KPPh2
About This Item
Recommended Products
form
liquid
reaction suitability
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: ligand
concentration
0.5 M in THF
density
0.929 g/mL at 25 °C
functional group
phosphine
SMILES string
[K]P(c1ccccc1)c2ccccc2
InChI
1S/C12H10P.K/c1-3-7-11(8-4-1)13-12-9-5-2-6-10-12;/h1-10H;/q-1;+1
InChI key
FCLYZQXPJKJTDR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Synthesis, reactivity and molecular structure of phosphino tetramethyl cyclopentadienyl complex (eta5: eta1-C5Me4CH2PPh2)Re(CO)2.: This study explores the synthesis and reactivity of a novel phosphino complex, utilizing Potassium diphenylphosphide as a key reagent. The research highlights the molecular structure and potential applications in organometallic chemistry (Godoy et al., 2009).
- Improved and efficient synthesis of chiral N,P-ligands via cyclic sulfamidates for asymmetric addition of butyllithium to benzaldehyde.: This paper details an improved synthesis method for chiral N,P-ligands using Potassium diphenylphosphide. The research demonstrates the compound′s efficacy in asymmetric synthesis, offering potential advancements in drug discovery and development (Rönnholm et al., 2007).
- Coordination chemistry of diselenophosphinate complexes: the X-ray single-crystal structures of [K(Se2PPh2)(THF)2]2 and [In(Se2PPh2)3].L (L = THF, PhMe).: This article investigates the coordination chemistry involving diselenophosphinate complexes, with Potassium diphenylphosphide playing a crucial role. The study provides detailed X-ray crystallographic data, offering insights for future research in chemical synthesis and materials science (Davies et al., 2004).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
-4.0 °F - closed cup
Flash Point(C)
-20 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service