363529
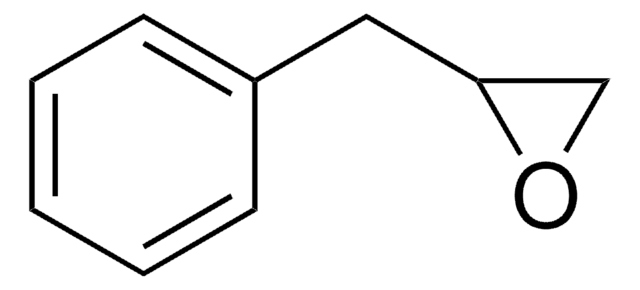
(R)-(−)-Glycidyl benzyl ether
99%
Synonym(s):
(R)-(−)-2-(Benzyloxymethyl)oxirane
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C10H12O2
CAS Number:
Molecular Weight:
164.20
Beilstein:
3588399
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
liquid
optical activity
[α]20/D −5.4°, c = 5 in toluene
refractive index
n20/D 1.517 (lit.)
density
1.077 g/mL at 25 °C (lit.)
functional group
ether
phenyl
storage temp.
2-8°C
SMILES string
C(OCc1ccccc1)[C@H]2CO2
InChI
1S/C10H12O2/c1-2-4-9(5-3-1)6-11-7-10-8-12-10/h1-5,10H,6-8H2/t10-/m0/s1
InChI key
QNYBOILAKBSWFG-JTQLQIEISA-N
Looking for similar products? Visit Product Comparison Guide
Application
Used in the preparation of the lactone fragment of compactin and mevinolin. Chiron in the preparation of syn-1,3-polyols, dideoxynucleosides, and a spiroacetal cyanohydrin.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis, 539-539 (1989)
Journal of the Chemical Society. Chemical Communications, 291-291 (1993)
E Abushanab et al.
Journal of medicinal chemistry, 32(1), 76-79 (1989-01-01)
1',2'-seco-2',3'-Dideoxycytidine (12), -guanosine (14), -adenosine (16), and -inosine (18) were prepared from (R)-benzylglycidol as potential anti-HIV agents. When compared to ddAdo in protecting ATH8 cells, they were found to be inactive.
The Journal of Organic Chemistry, 53, 4495-4495 (1988)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service