All Photos(1)
About This Item
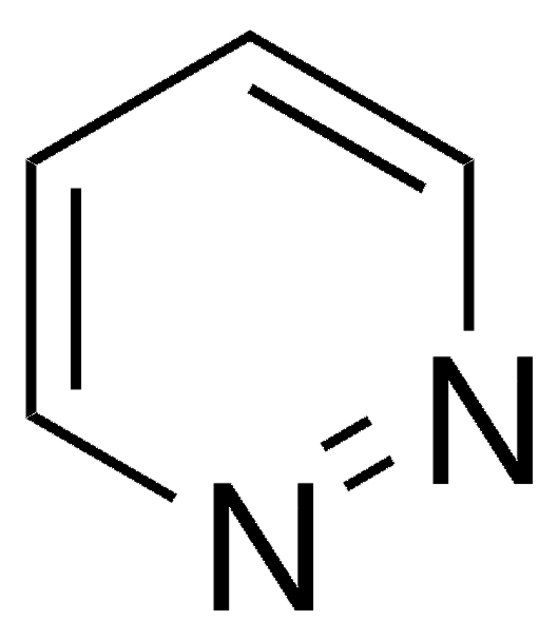
Empirical Formula (Hill Notation):
C5H6N2
CAS Number:
Molecular Weight:
94.11
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.521 (lit.)
bp
98-100 °C/11 mmHg (lit.)
density
1.06 g/mL at 25 °C (lit.)
SMILES string
Cc1ccnnc1
InChI
1S/C5H6N2/c1-5-2-3-6-7-4-5/h2-4H,1H3
InChI key
AIKUBOPKWKZULG-UHFFFAOYSA-N
General description
Products of reaction of methyl iodide with 4-methylpyridazine has been investigated by NMR spectra. Solvent free oxidation of 4-methylpyridazine by acetyl peroxyborate, peracetic acid and hydrogen peroxide has been reported.
Application
4-Methylpyridazine was used in the preparation of series of novel pyridazine derivatives structurally related to bipyridine cardiotonics.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
222.8 °F - closed cup
Flash Point(C)
106 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Investigation of the quaternisation products of 3-and 4-methylpyridazines by nuclear magnetic resonance spectroscopy.
Bale MS, et al.
Journal of the Chemical Society B: Physical Organic, 867-870 (1966)
N Haider et al.
Die Pharmazie, 44(9), 598-601 (1989-09-01)
Preparation of a series of novel pyridazine derivatives structurally related to bipyridine cardiotonics, starting from 4-methylpyridazine or 4-acetylpyridazine, respectively, is described. As observed with compounds 8, 11 and 16, an enhancement of in vitro cardiotonic activity is associated with the
Robert Raja et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 14(8), 2340-2348 (2008-01-30)
Niacin (3-picolinic acid), which is extensively used as vitamin B3 in foodstuffs and as a cholesterol-lowering agent, along with other oxygenated products of the picolines, 4-methylquinoline, and a variety of pyrimidines and pyridazines, may be produced in a single-step, environmentally
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service