All Photos(1)
About This Item
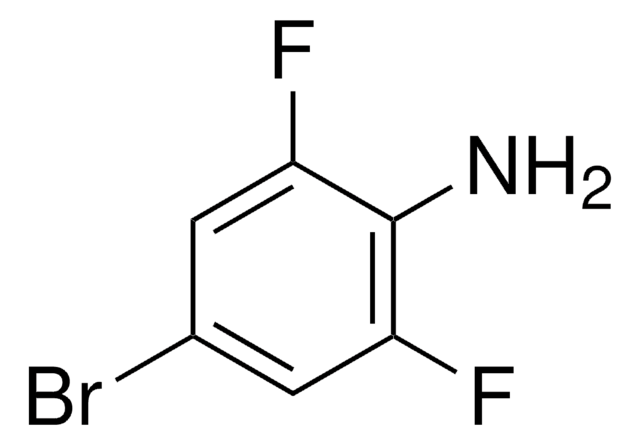
Linear Formula:
F2C6H2(NO2)NH2
CAS Number:
Molecular Weight:
174.10
Beilstein:
2723242
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
107-108 °C (lit.)
SMILES string
Nc1cc(F)c(F)cc1[N+]([O-])=O
InChI
1S/C6H4F2N2O2/c7-3-1-5(9)6(10(11)12)2-4(3)8/h1-2H,9H2
InChI key
WDMCABATCGQAKK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
4,5-Difluoro-2-nitroaniline has been used in the preparation of:
- 2-chloro-5,6-difluorobenzimidazole
- 1-(4,5-difluoro-2-nitrophenyl)pyrene via diazotization reaction with isoamyl nitrite in the presence of pyrene
- 5-ethoxy-6-fluorobenzofuroxan
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Novel substituted quinoxaline 1,4-dioxides with in vitro antimycobacterial and anticandida activity.
Antonio Carta et al.
European journal of medicinal chemistry, 37(5), 355-366 (2002-05-15)
Thirty-six 6(7)-substituted-3-methyl- or 3-halogenomethyl-2-phenylthio-phenylsulphonyl-chloro-quinoxaline 1,4-dioxides belonging to series 3-6 were synthesised and submitted to a preliminary in vitro evaluation for antimycobacterial, anticandida and antibacterial activities. Antitubercular screening showed a generally good activity of 3-methyl-2-phenylthioquinoxaline 1,4-dioxides (3d,e,h-j) against Mycobacterium tuberculosis, and
Synthesis of fluorinated derivatives of benzo [k] fluoranthene and indeno [1, 2, 3-cd] pyrene and 8, 9-dihydro-8, 9-epoxybenzo [k] fluoranthene.
Rice JE, et al.
The Journal of Organic Chemistry, 53(8), 1775-1779 (1988)
R Zou et al.
Journal of medicinal chemistry, 40(5), 811-818 (1997-02-28)
2-Chloro-5,6-difluorobenzimidazole (8) was prepared from 4,5-difluoro-2-nitroaniline (5) via successive reduction, cyclization, and diazotization reactions. 2-Chloro-5,6-dibromobenzimidazole (10) was obtained by a direct bromination of 2-chlorobenzimidazole (9) with bromine-water. 2-Chloro-5,6-diiodobenzimidazole (15) was synthesized by a stepwise transformation of the nitro functions of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)




![4,7-Dibromobenzo[c]-1,2,5-thiadiazole 95%](/deepweb/assets/sigmaaldrich/product/structures/711/964/3fd3ffd1-5916-468e-a743-22f1611b5a33/640/3fd3ffd1-5916-468e-a743-22f1611b5a33.png)