All Photos(1)
About This Item
Linear Formula:
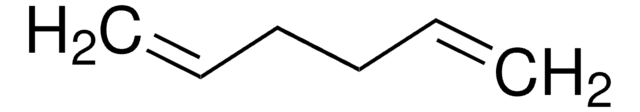
CH2=CH(CH2)3CH=CH2
CAS Number:
Molecular Weight:
96.17
Beilstein:
1731760
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
liquid
refractive index
n20/D 1.414 (lit.)
bp
89-90 °C (lit.)
mp
−129.4-−129 °C (lit.)
density
0.714 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C=CCCCC=C
InChI
1S/C7H12/c1-3-5-7-6-4-2/h3-4H,1-2,5-7H2
InChI key
GEAWFZNTIFJMHR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Highly stereoselective and regiospecific living coordination polymerization of 1,6-heptadiene has been used to generate a spectrum of different physical forms of poly(1,3-methylenecyclohexane).
Application
1,6-Heptadiene has been used as a starting reagent in asymmetric synthesis of all stereoisomers of 6-methylpipecolic acids.
accessory
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Asp. Tox. 1 - Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
15.8 °F - closed cup
Flash Point(C)
-9 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kaitlyn E Crawford et al.
Journal of the American Chemical Society, 135(24), 8778-8781 (2013-05-23)
External control over the rate of dynamic methyl group exchange between configurationally stable active species and configurationally unstable dormant species with respect to chain-growth propagation within a highly stereoselective and regiospecific living coordination polymerization of 1,6-heptadiene has been used to
H Takahata et al.
Amino acids, 24(3), 267-272 (2003-04-23)
Asymmetric synthesis of all four stereoisomers of 6-methylpipecolic acids with high enantiomeric purity via iterative AD reaction, starting from 1,6-heptadiene, has been described.
Roda Seseogullari-Dirihan et al.
Dental materials journal, 37(3), 445-452 (2018-03-02)
The aim of this study was to evaluate the effect of curcuminoids on the dentin endogenous protease activity. Demineralized dentin were pretreated with 50 or 100 µM of three different curcuminoids for 60 s and incubated up to 3 months.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service