All Photos(1)
About This Item
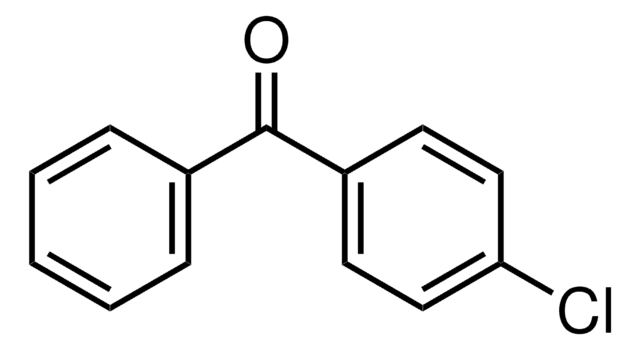
Linear Formula:
ClC6H4COC6H5
CAS Number:
Molecular Weight:
216.66
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥99%
bp
330 °C (lit.)
mp
44-47 °C (lit.)
SMILES string
Clc1ccccc1C(=O)c2ccccc2
InChI
1S/C13H9ClO/c14-12-9-5-4-8-11(12)13(15)10-6-2-1-3-7-10/h1-9H
InChI key
VMHYWKBKHMYRNF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Thermodynamics of formation of inclusion complex between 2-chlorobenzophenone and cyclomaltoheptaose (β-cyclodextrin) has been investigated by UV-vis spectroscopy and reversed-phase liquid chromatography. 2-Chlorobenzophenone undergoes reduction in the presence of LiAlH4 and (R)-(-)-2-(2-iso-indolinyl)butan-1-ol to afford the corresponding benzhydrols.
Application
2-Chlorobenzophenone was used in the synthesis of 1-(2-chlorophenyl)isoquinolin-3-yl trifluoromethanesulfonate.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Matias I Sancho et al.
Carbohydrate research, 346(13), 1978-1984 (2011-06-07)
A thermodynamic study of the inclusion process between 2-chlorobenzophenone (2ClBP) and cyclomaltoheptaose (β-cyclodextrin, β-CD) was performed using UV-vis spectroscopy, reversed-phase liquid chromatography (RP-HPLC), and molecular modeling (PM6). Spectrophotometric measurements in aqueous solutions were performed at different temperatures. The stoichiometry of
Synthesis of N-methyl-N-(1-methylpropyl)-1-(2-chlorophenyl) isoquinoline-3-[11 C] carboxamide ([11 C-carbonyl] PK11195) and some analogues using [11 C] carbon monoxide and 1-(2-chlorophenyl) isoquinolin-3-yl triflate.
Rahman O, et al.
Journal of the Chemical Society. Perkin Transactions 1, 23, 2699-2703 (2002)
Asymmetric reductions of ketones using lithium aluminium hydride modified with N, N-dialkyl derivatives of (R)-(-)-2-aminobutan-1-ol.
Brown E, et al.
Tetrahedron Asymmetry, 2(5), 339-342 (1991)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service