All Photos(2)
About This Item
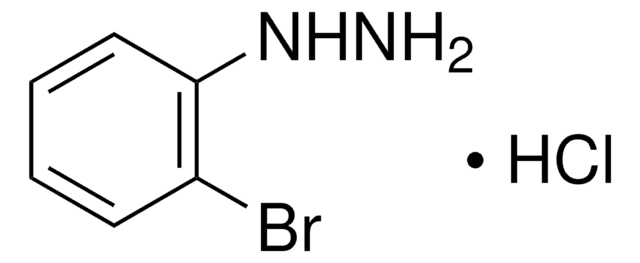
Linear Formula:
ClC6H4NHNH2·HCl
CAS Number:
Molecular Weight:
179.05
Beilstein:
3699381
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
200-203 °C (dec.) (lit.)
SMILES string
Cl.NNc1ccccc1Cl
InChI
1S/C6H7ClN2.ClH/c7-5-3-1-2-4-6(5)9-8;/h1-4,9H,8H2;1H
InChI key
ADODRSVGNHNKAT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2-Chlorophenylhydrazine hydrochloride was used to study intrinsic resistance of Mycobacterium smegmatis to fluoroquinolones, used for treatment of Mycobacterium tuberculosis. It may be used in pyrazoline synthesis.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Acute 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Mohammad Abdel-Halim et al.
Bioorganic & medicinal chemistry, 21(23), 7343-7356 (2013-10-22)
Derivatives with scaffolds of 1,3,5-tri-substituted pyrazoline and 1,3,4,5-tetra-substituted pyrazoline were synthesized and tested for their inhibitory effects versus the p53(+/+) HCT116 and p53(-/-) H1299 human tumor cell lines. Several compounds were active against the two cell lines displaying IC50 values
C Montero et al.
Antimicrobial agents and chemotherapy, 45(12), 3387-3392 (2001-11-16)
The fluoroquinolones (FQ) are used in the treatment of Mycobacterium tuberculosis, but the development of resistance could limit their effectiveness. FQ resistance (FQ(R)) is a multistep process involving alterations in the type II topoisomerases and perhaps in the regulation of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service