E29125
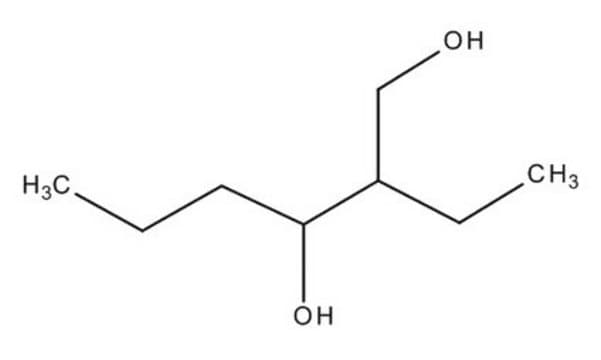
2-Ethyl-1,3-hexanediol
97%, Mixture of isomers
Synonym(s):
Ethylhexylene glycol
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CH3CH2CH2CH(OH)CH(C2H5)CH2OH
CAS Number:
Molecular Weight:
146.23
Beilstein:
1735324
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
5 (vs air)
Quality Level
Assay
97%
refractive index
n20/D 1.451 (lit.)
bp
241-249 °C (lit.)
mp
−40 °C (lit.)
density
0.933 g/mL at 25 °C (lit.)
SMILES string
CCCC(O)C(CC)CO
InChI
1S/C8H18O2/c1-3-5-8(10)7(4-2)6-9/h7-10H,3-6H2,1-2H3
InChI key
RWLALWYNXFYRGW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
2-Ethyl-1,3-hexanediol (EHD) can be used as:
- A boron extractant.
- A reactive solvent in the synthesis of magnetic iron-oxide nanoparticles by non-hydrolytic sol-gel method.
- A starting material in the selective synthesis of 2-ethyl-1-hydroxy-3-hexanone by oxidation using H2O2.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
276.8 °F - closed cup
Flash Point(C)
136 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Structural characterization and functional correlation of Fe3O4 nanocrystals obtained using 2-ethyl-1, 3-hexanediol as innovative reactive solvent in non-hydrolytic sol-gel synthesis
Sciancalepore C, et al.
Materials Chemistry and Physics, 207(0), 337-349 (2018)
Linda Ritzen et al.
Polymers, 13(2) (2021-01-23)
The use of self-healing (SH) polymers to make 3D-printed polymeric parts offers the potential to increase the quality of 3D-printed parts and to increase their durability and damage tolerance due to their (on-demand) dynamic nature. Nevertheless, 3D-printing of such dynamic
R S Slesinski et al.
Toxicology, 53(2-3), 179-198 (1988-12-30)
2-Ethyl-1,3-hexanediol (EHD) has intentional human exposure because of its application to skin as an insect repellent and its use in various skin care products. Genotoxicity studies on EHD were conducted to determine mutagenic and clastogenic potential using in vitro and
P Couch et al.
American journal of hospital pharmacy, 49(5), 1164-1173 (1992-05-01)
Lyme disease and the use of tick repellents and physical protective measures to prevent the disease are discussed. Lyme disease is a multiple-organ-system, immune-mediated inflammatory disorder transmitted by the bites of ixodid ticks infected with Borrelia burgdorferi. An individual is
Z A Mehr et al.
Journal of the American Mosquito Control Association, 6(3), 469-476 (1990-09-01)
Studies by prior workers have shown that insect repellents can act as attractants when present as low concentrations, deposits or residues. In the present study deet and ethyl hexanediol were tested in 2-fold serial doses from 1.9 X 10(-9) to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service