All Photos(1)
About This Item
Empirical Formula (Hill Notation):
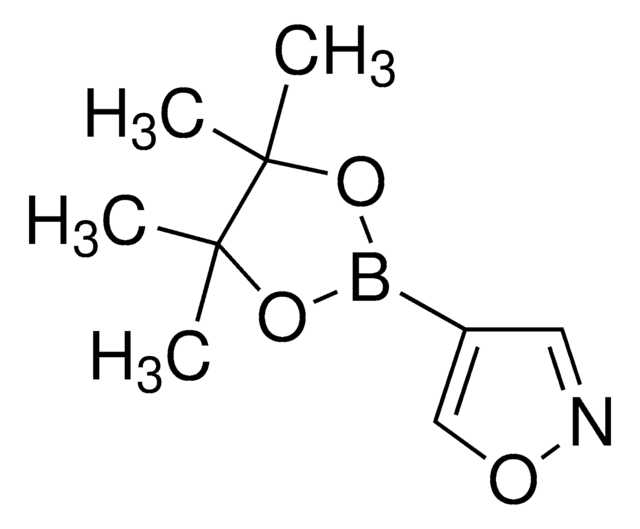
C14H17BN2O2
CAS Number:
Molecular Weight:
256.11
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
refractive index
n20/D 1.560
density
1.123 g/mL at 25 °C
SMILES string
CC1(C)OB(OC1(C)C)c2ccc3nccnc3c2
InChI
1S/C14H17BN2O2/c1-13(2)14(3,4)19-15(18-13)10-5-6-11-12(9-10)17-8-7-16-11/h5-9H,1-4H3
InChI key
ZYWICCYXTGRUNM-UHFFFAOYSA-N
Application
Quinoxaline-6-boronic acid pinacol ester is a common reactant of a Suzuki coupling reaction that can be used:
- To prepare quinoxalin based PI3Kδ inhibitors.
- As a substrate in the Cu(II) catalyzed [11C]-radiocyanation of arylboronic acids.
- To prepare 6-quinoxaline boronic acid, which is used as a substrate in the silver-mediated fluorination of boronic acids.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
>230.0 °F
Flash Point(C)
> 110 °C
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Fluorination of boronic acids mediated by silver (I) triflate
Furuya T and Ritter T
Organic Letters, 11(13), 2860-2863 (2009)
Copper (II)-mediated [11C] cyanation of arylboronic acids and arylstannanes
Makaravage KJ, et al.
Organic Letters, 20(6), 1530-1533 (2018)
Design of selective PI3Kδ inhibitors using an iterative scaffold-hopping workflow
Fradera X, et al.
Bioorganic & Medicinal Chemistry Letters, 29(18), 2575-2580 (2019)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service