All Photos(1)
About This Item
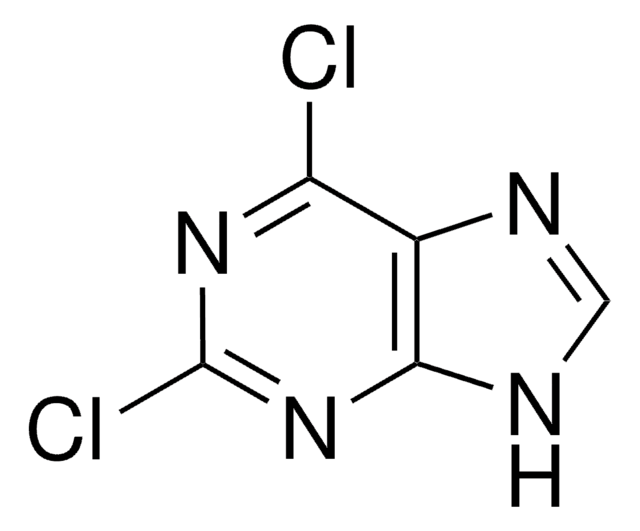
Empirical Formula (Hill Notation):
C5H3ClN4
CAS Number:
Molecular Weight:
154.56
Beilstein:
5774
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
mp
>300 °C (dec.) (lit.)
functional group
chloro
SMILES string
Clc1ncnc2[nH]cnc12
InChI
1S/C5H3ClN4/c6-4-3-5(9-1-7-3)10-2-8-4/h1-2H,(H,7,8,9,10)
InChI key
ZKBQDFAWXLTYKS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
6-Chloropurine (6-CIPH), a 6-substituted purine derivative, is an antileukemic drug. It can be prepared by the chlorination of hypoxanthine with phosphorus oxychloride in the presence of dimethylaniline. The NMR-based conformational analysis of the products formed during the reaction of 6-CIPH with 3,4-di-O-acetyl-D-xylal and 3,4-di-O-acetyl-L-arabinal have been reported. 6-CIPH can undergo palladium-catalyzed cross coupling with organostannanes at 6-position to form the corresponding arylated or alkylated products.
Application
6-Chloropurine may be used:
- To prepare purine via catalytic dehydrogenation.
- To prepare 9-alkylated adenines via Mitsunobu reaction with various alcohols.
- As a starting material to synthesize dihydroisoxazole 6-chloropurine.
Signal Word
Warning
Hazard Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
REACTIONS OF RIBONUCLEOTIDE DERIVATIVES OF PURINE ANALOGUES AT THE CATALYTIC SITE OF INOSINE 5'-PHOSPHATE DEHYDROGENASE.
A HAMPTON
The Journal of biological chemistry, 238, 3068-3074 (1963-09-01)
The Synthesis and Properties of 6-Chloropurine and Purine1.
Bendich A, et al.
Journal of the American Chemical Society, 76(23), 6073-6077 (1954)
Synthesis and anti-HIV activity of dihydroisoxazole 6-chloropurine and adenine.
Xiang Y, et al.
Bioorganic & Medicinal Chemistry Letters, 6(9), 1051-1054 (1996)
Synthesis of Nucleosides and Related Compounds. Part XXV. The Alkylation of 6-Chloropurine with Alcohols by Mitsunobu Reaction.
Toyota A, et al.
Chemical & Pharmaceutical Bulletin, 40(4), 1039-1041 (1992)
6-Chloropurines and organostannanes in palladium catalyzed cross coupling reactions.
Gundersen LL.
Tetrahedron Letters, 35(19), 3155-3158 (1994)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service