All Photos(1)
About This Item
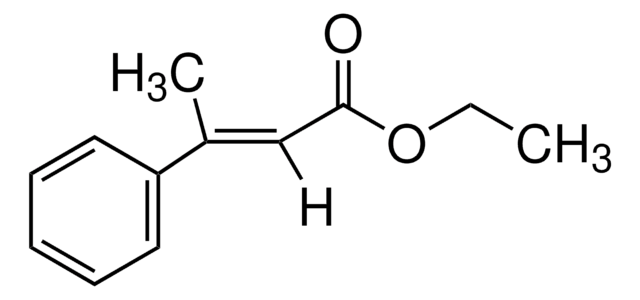
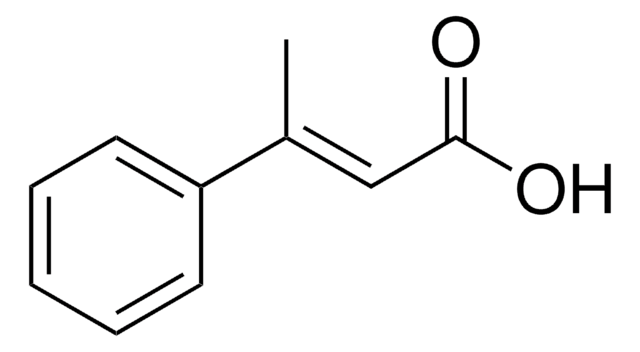
Linear Formula:
CH3C6H4CH=CHCO2H
CAS Number:
Molecular Weight:
162.19
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
mp
174-176 °C (lit.)
SMILES string
Cc1ccccc1\C=C\C(O)=O
InChI
1S/C10H10O2/c1-8-4-2-3-5-9(8)6-7-10(11)12/h2-7H,1H3,(H,11,12)/b7-6+
InChI key
RSWBWHPZXKLUEX-VOTSOKGWSA-N
General description
2-Methylcinnamic acid has been reported to exhibit strong anti-fungal activity against white-rot fungus Lenzites betulina and brown-rot fungus Laetiporus sulphureus. Hydrogenation of 2-methylcinnamic acid using Walphos ligands and their biferrocene analogs has been studied.
Application
2-Methylcinnamic acid may be used as starting reagent for the total synthesis of the cytotoxic alkaloid, 22-hydroxyacuminatine and for the preparation of (E)-2-methylcinnamic acid i-butylammonium salt.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Xiangshu Xiao et al.
Journal of medicinal chemistry, 49(4), 1408-1412 (2006-02-17)
A total synthesis of 22-hydroxyacuminatine, a cytotoxic alkaloid isolated from Camptotheca acuminata, is reported. The key step in the synthesis involves the reaction of 2,3-dihydro-1H-pyrrolo[3,4-b]quinoline with a brominated phthalide to generate a substituted pentacyclic 12H-5,11a-diazadibenzo[b,h]fluoren-11-one intermediate. Despite its structural resemblance
Sen-Sung Cheng et al.
Bioresource technology, 99(11), 5145-5149 (2007-10-20)
In this study, the antifungal activities of cinnamaldehyde and eugenol congeners against white-rot fungus Lenzites betulina and brown-rot fungus Laetiporus sulphureus were evaluated and the relationships between the antifungal activity and the chemical structures were also examined. Results from antifungal
Martin E Fox et al.
The Journal of organic chemistry, 73(3), 775-784 (2007-10-24)
Four chiral diphosphine ligands consisting of bis(2,5-diphenylphospholan-1-yl) groups connected by the sp(2) carbon linkers 2,3-quinoxaline ((S,S)-Ph-Quinox), 2,3-pyrazine ((S,S)-Ph-Pyrazine), maleic anhydride ((S,S)-Ph-MalPhos), and 1,1'-ferrocene ((S,S)-Ph-5-Fc) were synthesized, and their cationic [rhodium(I)(COD)] complexes were prepared. These complexes were tested in asymmetric hydrogenation
Afrooz Zirakzadeh et al.
Organometallics, 33(8), 1945-1952 (2014-05-06)
Two representative Walphos analogues with an achiral 2,2″-biferrocenediyl backbone were synthesized. These diphosphine ligands were tested in the rhodium-catalyzed asymmetric hydrogenation of several alkenes and in the ruthenium-catalyzed hydrogenation of two ketones. The results were compared with those previously obtained
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service