T7063
Tryptase from human lung
buffered aqueous solution, ≥5 units/mg protein
Synonym(s):
Tryptase enzyme
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
form
buffered aqueous solution
Quality Level
specific activity
≥5 units/mg protein
mol wt
~135 kDa
shipped in
dry ice
storage temp.
−20°C
Gene Information
human ... TPSAB1(7177) , TPSB2(64499) , TPSD1(23430) , TPSG1(25823)
General description
Tryptase is a glycoprotein released from mast cells during anaphylaxis, which performs a number of functions including catalyzing the activation of complement C3, converting prostromelysin to stromelysin (MMP-3), and cleaving fibrinogen resulting in a loss of clotting potential. Human tryptase is a major secretory protease of human lung mast cells.
Application
Tryptase has been used in a study that purified and characterized recombinant rat mast cell protease 7 expressed in Pichia pastoris. Tryptase has also been used in a study to investigate drug allergies in mast cell disease.
Biochem/physiol Actions
Tryptase is a member of the serine protease S1 family. It is the predominant neutral protease of the mast cell granules. Within the mast cell granule it exists as a heparin-stabilized active tetramer. Stabilization is a result of the high negative charge density of the glycosaminoglycan. This stabilization activity is observed with heparins with a MW greater than 6 kDa as well as other glycosaminoglycans such as dextran sulfate or chondroitin sulfates. Removal of heparin results in dissociation of the tetramer and inactivation of the enzyme. High concentrations of NaCl will result in the dissociation of heparin.
Tryptase is released from the mast cell as a result of the degranulation response during anaphylaxis. In addition, several tryptase genes and alleles (α, β, γ & δ) have been identified in various tissues and circulating in blood. Pro-β-tryptase is thought to be the constituative circulating form in blood.
The biological function of tryptase is unknown. However it has been reported to catalyze the activation of complement C3, convert prostromelysin to stromelysin (MMP-3), and cleave fibrinogen resulting in a loss of clottting potential. Tryptase also degrades fibronectin, calcitonin gene-related peptide, vasoactive intestinal peptide,
and kininogen.
Tryptase is released from the mast cell as a result of the degranulation response during anaphylaxis. In addition, several tryptase genes and alleles (α, β, γ & δ) have been identified in various tissues and circulating in blood. Pro-β-tryptase is thought to be the constituative circulating form in blood.
The biological function of tryptase is unknown. However it has been reported to catalyze the activation of complement C3, convert prostromelysin to stromelysin (MMP-3), and cleave fibrinogen resulting in a loss of clottting potential. Tryptase also degrades fibronectin, calcitonin gene-related peptide, vasoactive intestinal peptide,
and kininogen.
Quality
Highly purified
Physical properties
Molecular Weight: ~135 kDa (Human)(Non-covalently linked tetramer with two sets of dissimilar subunits possibly resulting from heterogeneity in N-linked glycosylation and existence of a & b isoforms sequences in human lung). 31-33 kDa (Monomer MW)
Unit Definition
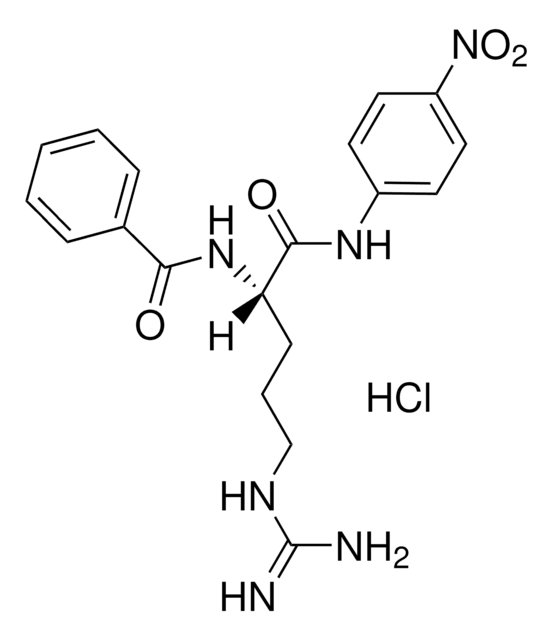
One unit will hydrolyze 1.0 μmole of N-benzyl-DL-Arg-pNA per minute at pH 8 at 25 °C.
Physical form
Supplied as a liquid in 50mM Sodium Acetate, 1M Sodium Chloride, pH 5.0 with 0.05mM Heparin and 0.01% Sodium Azide
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ayşe Baççıoğlu Kavut et al.
International archives of allergy and immunology, 160(2), 184-191 (2012-09-29)
A neuroallergic interaction was reported in the pathogenesis of allergic rhinitis (AR), but the pathophysiology of nonallergic rhinitis (NAR) is poorly understood. We aimed to explore the contribution of neuroallergic mechanisms to the pathogenesis of NAR. Subjects were divided into
Degradation of airway neuropeptides by human lung tryptase
Tam, E. and G. Caughey
American Journal of Respiratory Cell and Molecular Biology, 3, 37-32 (1990)
Weiqun Yu et al.
PloS one, 7(11), e48897-e48897 (2012-11-13)
Interstitial cells of Cajal (ICC) have been identified in urinary bladder of several species, but their presence in mice remains uncertain. Meanwhile, dozens of reports indicate that dysregulation of connexin 43 plays an important role in bladder overactivity, but its
Nicolas Guillaume et al.
The American journal of medicine, 126(1), 75-75 (2012-12-04)
Mastocytosis is a heterogeneous group of clonal mast cell disorders in which bone manifestations are frequently seen, but poorly understood. In this study, we analyzed correlation of clinical findings in mastocytosis patients with bone mineral density and bone turnover markers.
G F Stefanini et al.
European annals of allergy and clinical immunology, 44(4), 175-177 (2012-10-25)
A 39-years-old man afferred to our hospital for a fever lasting for more than 6 months, without abnormalities at physical examination (in particular no skin alterations); a recent laboratory and instrumental investigation was ineffective and so a fever of unknown
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service