iopamiron is not available but iodixanol, iopromide, and iohexol can be used as reference standards, which are likely more expensive on a per gram basis due to their intended use. The only other chemical close to iopamiron is ionic rather than non-ionic.
D9268
Diatrizoic acid
≥98% purity, powder
Synonym(s):
Amidotrizoic Acid, NSC 262168
Select a Size
About This Item
Recommended Products
Product Name
Diatrizoic acid, Iodine-containing contrast agent
Quality Level
form
powder
composition
Water (by Karl Fischer): ≤ 7.1%
concentration
≥98.0% AgNO3
technique(s)
titration: suitable
color
white to off-white
solubility
1 M NH4OH: 100 mg/mL, colorless
application(s)
diagnostic assay manufacturing
hematology
histology
storage temp.
room temp
SMILES string
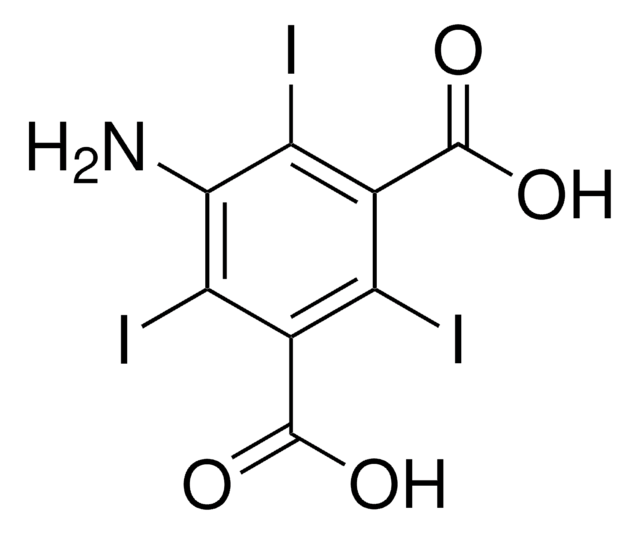
CC(=O)Nc1c(I)c(NC(C)=O)c(I)c(C(O)=O)c1I
InChI
1S/C11H9I3N2O4/c1-3(17)15-9-6(12)5(11(19)20)7(13)10(8(9)14)16-4(2)18/h1-2H3,(H,15,17)(H,16,18)(H,19,20)
InChI key
YVPYQUNUQOZFHG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
-
Are there any non-ionic iodinated contrast agents, such as Iopamiron?
1 answer-
Helpful?
-
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service