This product is a liquid at room temperature and will only become a solid at -21°C or colder. It is strongly recommended to review the Safety Data Sheet prior to working with this chemical. It is important to handle this chemical under an inert gas atmosphere, as it reacts violently with water and moisture in the air, likely causing the issues described. Additionally, it should be stored under inert gas.
562629
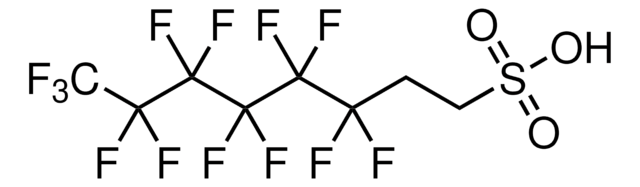
Nonafluorobutane-1-sulfonic acid
97%
Synonym(s):
1,1,2,2,3,3,4,4,4-Nonafluoro-1-butanesulfonic acid, 1-Perfluorobutanesulfonic acid, Nonafluoro-1-butanesulfonic acid, Nonafluorobutan-1-sulfonic acid, Nonafluorobutanesulfonic acid, Perfluorobutanesulfonic acid, n-Nonafluorobutanesulfonic acid
Select a Size
About This Item
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.3230 (lit.)
bp
112-114 °C/14 mmHg (lit.)
density
1.811 g/mL at 25 °C (lit.)
functional group
fluoro
sulfonic acid
storage temp.
2-8°C
SMILES string
OS(=O)(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F
InChI
1S/C4HF9O3S/c5-1(6,3(9,10)11)2(7,8)4(12,13)17(14,15)16/h(H,14,15,16)
InChI key
JGTNAGYHADQMCM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B
Supplementary Hazards
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
350.6 °F - Pensky-Martens closed cup
Flash Point(C)
177 °C - Pensky-Martens closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
-
1. 이 제품은 액체로 되어있습니다. 표준원액을 만들어야 하는데 피펫으로 끌어올렸을때 하얗게 굳습니다. 굳기전에 재빠르게 무게를 재서 표준원액을 조제하면 농도가 잘 맞지 않습니다. 어떻게 조제하는 것이 정확할까요? 2. 뚜껑을 열었을 때 연기가 나오는데 어떤 현상이 있는건가요 조심해야하나요? 2. 메탄올에 녹이는 것이 좋을까요?
1 answer-
Helpful?
-
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service