All Photos(1)
About This Item
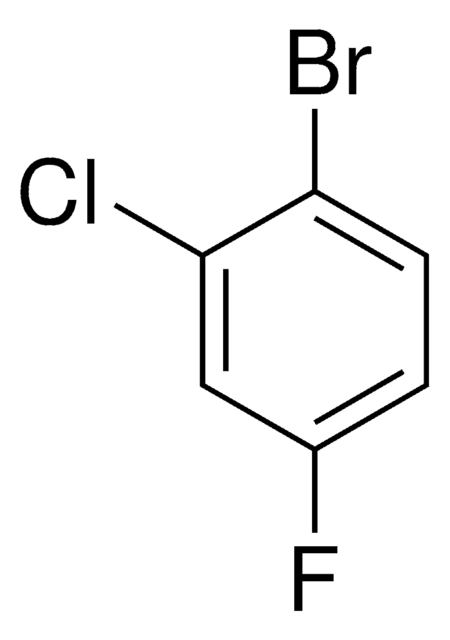
Linear Formula:
BrC6H3(Cl)F
CAS Number:
Molecular Weight:
209.44
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
refractive index
n20/D 1.556 (lit.)
bp
91-92 °C/20 mmHg (lit.)
density
1.678 g/mL at 25 °C (lit.)
SMILES string
Fc1cc(Cl)ccc1Br
InChI
1S/C6H3BrClF/c7-5-2-1-4(8)3-6(5)9/h1-3H
InChI key
FPNVMCMDWZNTEU-UHFFFAOYSA-N
General description
1-Bromo-4-chloro-2-fluorobenzene is a polyhalo substituted benzene. It undergoes Suzuki coupling with 2-cyanoarylboronic esters to form the corresponding biphenyls. These biphenyls are the precursors for synthesizing 6-substituted phenanthridines.† The enthalpy of vaporization at boiling point (467.15K) of 1-bromo-4-chloro-2-fluorobenzene is 40.737kjoule/mol.{4}
Application
1-Bromo-4-chloro-2-fluorobenzene may be used in the preparation of benzonorbornadiene derivative. It may also be used as a starting material in the multi-step synthesis of AZD3264, an IKK2 (inhibitor of nuclear factor κ-B kinase-2) inhibitor.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
198.0 °F - closed cup
Flash Point(C)
92.2 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of substituted 2-cyanoarylboronic esters.
Lysen M, et al.
The Journal of Organic Chemistry, 71(6), 2518-2520 (2006)
Exploiting the Differential Reactivities of Halogen Atoms: Development of a Scalable Route to IKK2 Inhibitor AZD3264
Murugan A, et al.
Organic Process Research & Development, 18(5), 646-651 (2014)
K C Caster et al.
The Journal of organic chemistry, 66(9), 2932-2936 (2001-04-28)
This report details the synthesis of several benzonorbornadienes by Diels--Alder cycloaddition of cyclopentadiene derivatives with substituted benzyne intermediates, which were generated by low-temperature metal--halogen exchange of halobenzenes. General conditions were developed, allowing synthesis of most benzonorbornadienes described herein at the
Thermophysical Properties of Chemicals and Hydrocarbons, 435-435 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service