345296
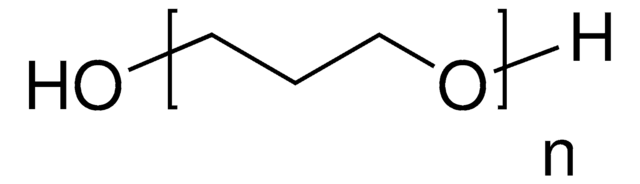
Poly(tetrahydrofuran)
average Mn ~1,000
Synonym(s):
α-Hydro-ω-hydroxypoly(oxy-1,4-butanediyl), Poly(1,4-butanediol), polyTHF
About This Item
Recommended Products
vapor pressure
<0.01 mmHg ( 25 °C)
<1 mmHg ( 20 °C)
mol wt
average Mn ~1,000
contains
0.05-0.07% BHT as stabilizer
mp
25-33 °C
density
0.974 g/mL at 25 °C
SMILES string
OCCCCO
InChI
1S/C8H18O2/c1-3-5-6-10-8(4-2)7-9/h8-9H,3-7H2,1-2H3/t8-/m0/s1
InChI key
BJZYYSAMLOBSDY-QMMMGPOBSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Thermally reversible urethane -epoxy networks.
- Poly(hexamethylene 2,6-naphthalate)-block-poly(tetrahydrofuran) (PHN-b-N-pTHF) copolymers with shape memory effect via melt polycondensation.
- Poly(3,4-ethylenedioxythiophene):poly(tetrahydrofuran) composite for the fabrication of memory organic electrochemical transistors.
Features and Benefits
- High flexibility
- Hydrolytic stability
- Excellent abrasion resistance
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
>325.4 °F - Tag open cup
Flash Point(C)
> 163 °C - Tag open cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service