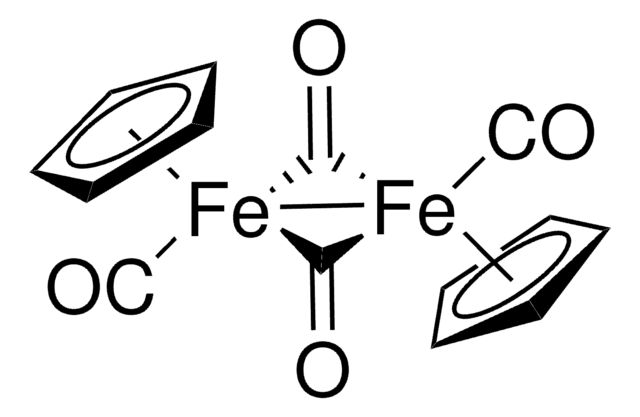
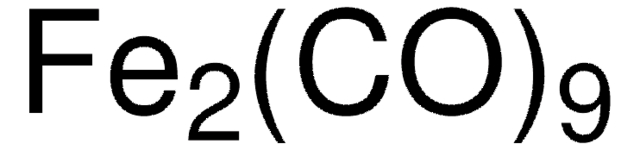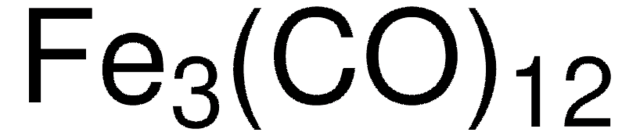This product is not tested for purity and is considered a general use item. Should the purity and additional analytical testing be required, see product 481718. A link to this product page, as well as a sample Certificate of Analysis are provided below:
481718 Iron(0) Pentacarbonyl product page:
https://www.sigmaaldrich.com/US/en/product/aldrich/481718
Sample Certificate of Analysis:
https://www.sigmaaldrich.com/certificates/sapfs/PROD/sap/certificate_pdfs/COFA/Q14/481718-BULKSHBQ5322.pdf