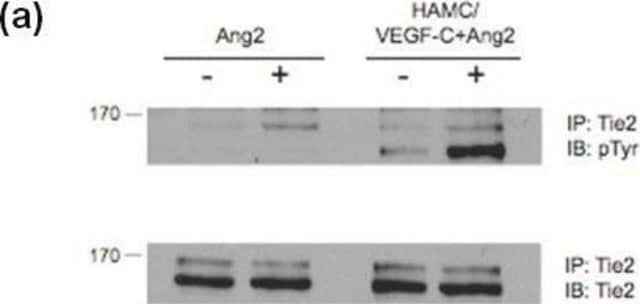
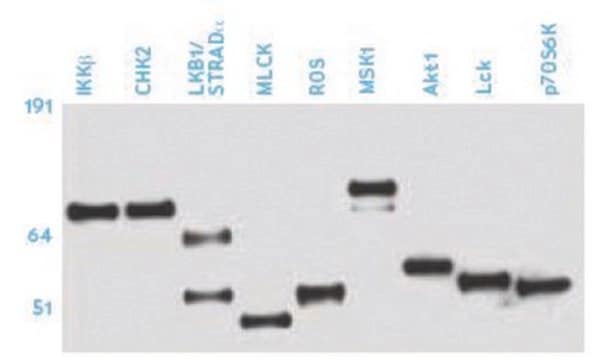
P6623
Anti-Phosphothreonine antibody, Mouse monoclonal
clone PTR-8, purified from hybridoma cell culture
Synonym(e):
Monoclonal Anti-Phosphothreonine, Phospho Thr, Phospho Threonine, Phospho-Thr, Phospho-Threonine, p-Thr
About This Item
Empfohlene Produkte
Biologische Quelle
mouse
Qualitätsniveau
Konjugat
unconjugated
Antikörperform
purified immunoglobulin
Antikörper-Produkttyp
primary antibodies
Klon
PTR-8, monoclonal
Form
buffered aqueous solution
Konzentration
~2.5 mg/mL
Methode(n)
dot blot: suitable
immunocytochemistry: suitable
immunoprecipitation (IP): suitable
indirect ELISA: 0.5-1 μg/mL
microarray: suitable
western blot: 5-10 μg/mL using A431 cell extracts
Isotyp
IgG2b
Versandbedingung
dry ice
Lagertemp.
−20°C
Posttranslationale Modifikation Target
unmodified
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Spezifität
Immunogen
Anwendung
Biochem./physiol. Wirkung
Physikalische Form
Lagerung und Haltbarkeit
Haftungsausschluss
Not finding the right product?
Try our Produkt-Auswahlhilfe.
Lagerklassenschlüssel
10 - Combustible liquids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.