BCR272
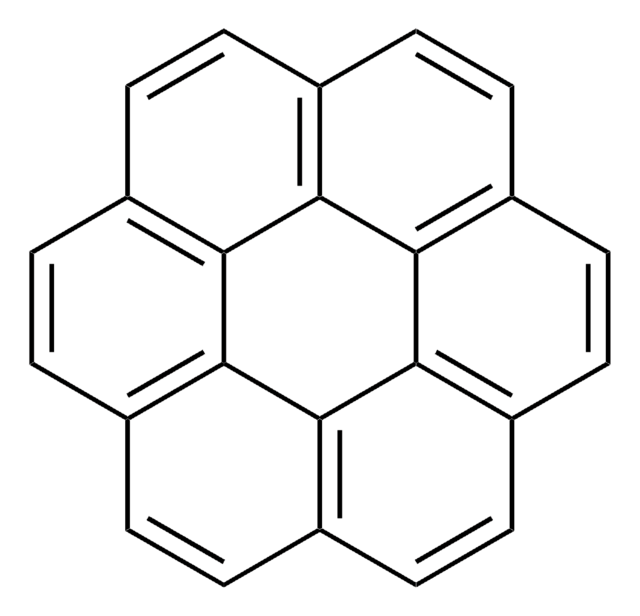
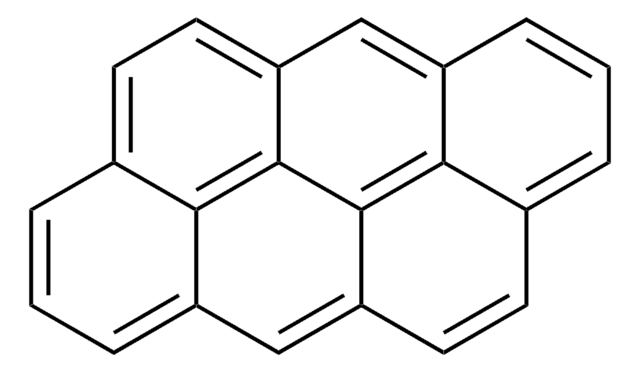
Coronen
BCR®, certified reference material
About This Item
Empfohlene Produkte
Qualität
certified reference material
Agentur
BCR®
Hersteller/Markenname
JRC
Methode(n)
HPLC: suitable
gas chromatography (GC): suitable
bp
525 °C (lit.)
mp (Schmelzpunkt)
428 °C (lit.)
Format
neat
Lagertemp.
2-8°C
SMILES String
c1cc2ccc3ccc4ccc5ccc6ccc1c7c2c3c4c5c67
InChI
1S/C24H12/c1-2-14-5-6-16-9-11-18-12-10-17-8-7-15-4-3-13(1)19-20(14)22(16)24(18)23(17)21(15)19/h1-12H
InChIKey
VPUGDVKSAQVFFS-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Hinweis zur Analyse
BCR272
Rechtliche Hinweise
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Analysenzertifikate (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Kundensupport
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.