240877
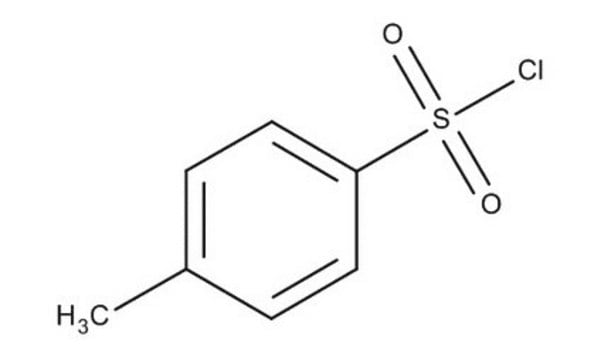
p-Toluolsulfonylchlorid
ReagentPlus®, ≥99%
Synonym(e):
Tosylchlorid
About This Item
Empfohlene Produkte
Dampfdruck
1 mmHg ( 88 °C)
Qualitätsniveau
Produktlinie
ReagentPlus®
Assay
≥99%
Form
solid
bp
134 °C/10 mmHg (lit.)
mp (Schmelzpunkt)
65-69 °C (lit.)
Löslichkeit
benzene: freely soluble(lit.)
chloroform: freely soluble(lit.)
ethanol: freely soluble(lit.)
water: insoluble(lit.)
SMILES String
Cc1ccc(cc1)S(Cl)(=O)=O
InChI
1S/C7H7ClO2S/c1-6-2-4-7(5-3-6)11(8,9)10/h2-5H,1H3
InChIKey
YYROPELSRYBVMQ-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
Anwendung
- In combination with N-methylimidazole for the esterification or thioesterification of carboxylic acids and alcohols or thiols.
- As an additive to enhance the yield of symmetrical biaryls via palladium chloride catalyzed homo-coupling of arylboronic acids in the absence of ligands.
- As a positive chlorine source for the ?-chlorination of ketones.
- Solvent-free tosylation of alcohols and phenols in the presence of heterodoxy acids.
- As an activator for reaction between 2-alkynylbenzaldoxime and phenols to form 1-aroxyisoquinolines in the presence of silver triflate.
- As a catalyst for the solvent-free preparation of symmetrical bis(benzhydryl)ethers from benzhydrols.
Rechtliche Hinweise
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Eye Dam. 1 - Met. Corr. 1 - Skin Irrit. 2 - Skin Sens. 1
Lagerklassenschlüssel
8B - Non-combustible corrosive hazardous materials
WGK
WGK 1
Flammpunkt (°F)
262.4 °F - closed cup
Flammpunkt (°C)
128 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
Click chemistry, and the copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) in particular, is a powerful new synthetic tool in polymer chemistry and material science.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.