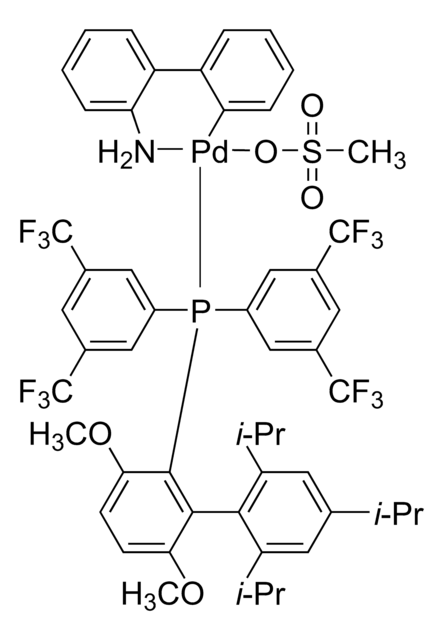
799718
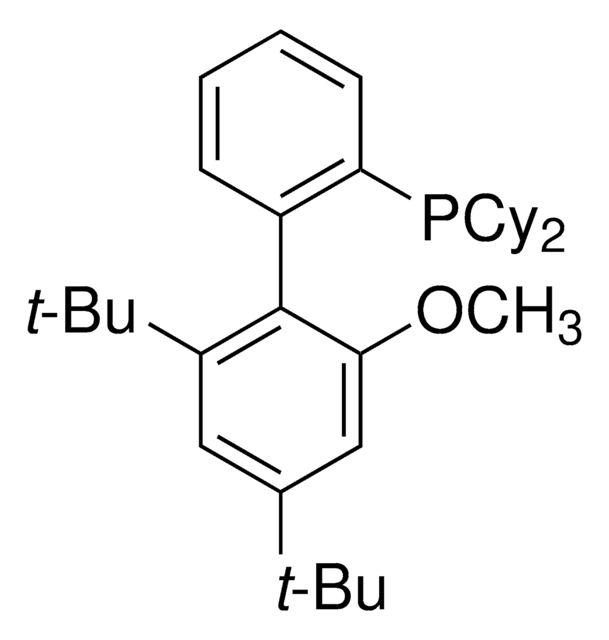
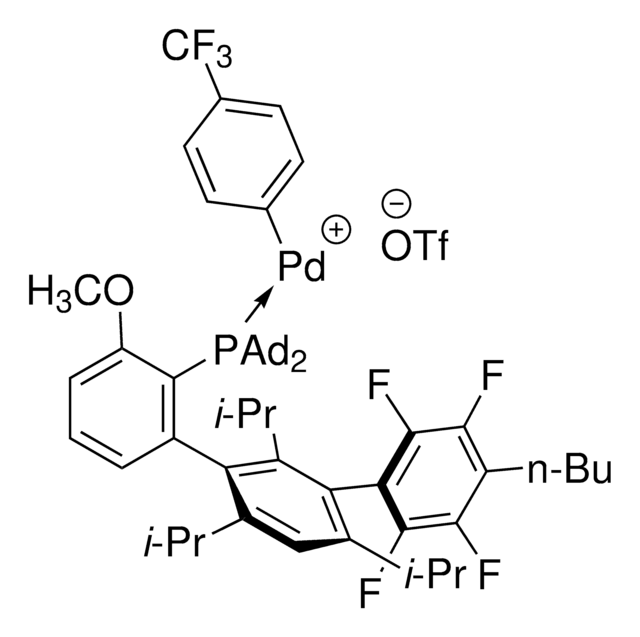
AlPhos
Synonym(e):
Di-1-adamantyl(4″-butyl-2″,3″,5″,6″-tetrafluoro-2′,4′,6′-triisopropyl-2-methoxy-meta-terphenyl)phosphine
About This Item
Empfohlene Produkte
Assay
≥95%
Qualitätsniveau
Form
powder
Eignung der Reaktion
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: ligand
reaction type: Cross Couplings
mp (Schmelzpunkt)
218-223 °C
Funktionelle Gruppe
phosphine
Lagertemp.
−20°C
InChI
1S/C52H67F4OP/c1-9-10-12-38-46(53)48(55)45(49(56)47(38)54)44-40(29(4)5)21-39(28(2)3)43(42(44)30(6)7)37-13-11-14-41(57-8)50(37)58(51-22-31-15-32(23-51)17-33(16-31)24-51)52-25-34-18-35(26-52)20-36(19-34)27-52/h11,13-14,21,28-36H,9-10,12,15-20,22-27H2,1-8H3/t31-,32+,33?,34-,35+,36?,51+,52?,58?
InChIKey
ALWIRDZSIXWCBO-VABCSHEKSA-N
Verwandte Kategorien
Anwendung
- In the Pd-catalyzed Buchwald-Hartwig cross-coupling reactions.
- To synthesize highly regioselective aryl fluorides by Pd-catalyzed fluorination of a variety of activated aryl and heteroaryl triflates and bromides.
- To prepare aryl thioethers by C–S cross-coupling of thiols with aromatic electrophile in the presence of palladium catalyst.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
Fluorine containing aromatics (ArF) are desirable compounds with applications in medicinal chemistry and the agricultural industry.
Verwandter Inhalt
The Buchwald group has developed a series of highly active and versatile palladium precatalysts and biarylphosphine ligands used in cross-coupling reactions for the formation of C-C, C–N, C–O, C–F, C–CF3, and C–S bonds. The ligands are electron-rich, and highly tunable to provide catalyst systems with a diverse scope, high stability and reactivity. Furthermore, the new series of precatalysts are air-, moisture and thermally-stable and display good solubility in common organic solvents. The use of precatalysts ensures the efficient generation of the active catalytic species and allows one to accurately adjust the ligand:palladium ratio. The ligands, precatalysts and methodology developed in the Buchwald group are user friendly and have rendered previously difficult cross couplings reactions, much easier to achieve.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.


![2-{Bis-[3,5-bis-(trifluoromethyl)-phenyl]-phosphino}-3,6-dimethoxy-2′,4′,6′-triisopropyl-1,1′-biphenyl 95%](/deepweb/assets/sigmaaldrich/product/structures/371/999/7169f55f-ccbe-4696-bbdb-ab785b331b2e/640/7169f55f-ccbe-4696-bbdb-ab785b331b2e.png)