532363
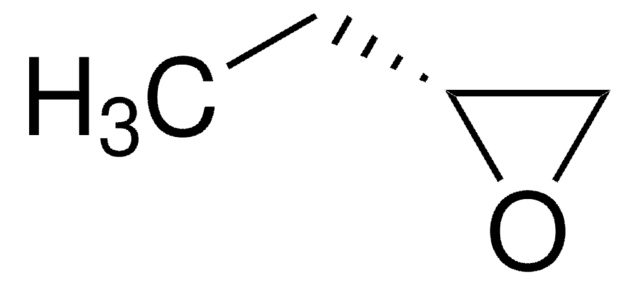
(S)-(−)-1,2-Epoxybutan
98%
Synonym(e):
(2S)-Ethyloxiran
About This Item
Empfohlene Produkte
Assay
98%
Optische Aktivität
[α]20/D −10°, neat
Brechungsindex
n20/D 1.386 (lit.)
bp
63 °C (lit.)
Dichte
0.837 g/mL at 25 °C (lit.)
SMILES String
CC[C@H]1CO1
InChI
1S/C4H8O/c1-2-4-3-5-4/h4H,2-3H2,1H3/t4-/m0/s1
InChIKey
RBACIKXCRWGCBB-BYPYZUCNSA-N
Verwandte Kategorien
Anwendung
- As a starting material to prepare (+)- and (−)-homononactic acids, which are used as intermediates in the total synthesis of a cyclic antibiotic tetranactin.
- To prepare a chiral phosphorus synthon, which is applicable in the synthesis of phytoprostane B1 type I.
- To prepare Eu3+-based precatalysts applicable in the Mukaiyama Aldol reaction in water.
Rechtliche Hinweise
Signalwort
Danger
Gefahreneinstufungen
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B
Lagerklassenschlüssel
3 - Flammable liquids
WGK
WGK 3
Flammpunkt (°F)
10.0 °F - closed cup
Flammpunkt (°C)
-12.2 °C - closed cup
Persönliche Schutzausrüstung
Faceshields, Gloves, Goggles
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.