Alle Fotos(1)
Wichtige Dokumente
347051
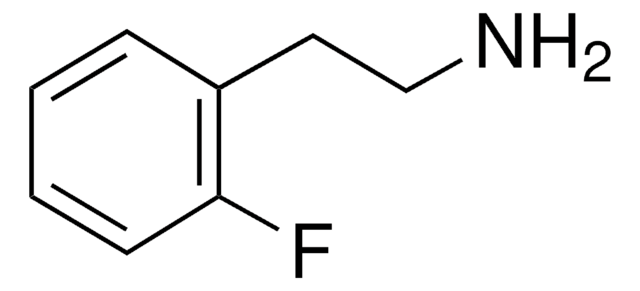
3-Fluorphenethylamin
99%
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Lineare Formel:
FC6H4CH2CH2NH2
CAS-Nummer:
Molekulargewicht:
139.17
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Assay
99%
Form
liquid
Brechungsindex
n20/D 1.509 (lit.)
bp
87 °C/15 mmHg (lit.)
Dichte
1.066 g/mL at 25 °C (lit.)
Funktionelle Gruppe
amine
fluoro
SMILES String
NCCc1cccc(F)c1
InChI
1S/C8H10FN/c9-8-3-1-2-7(6-8)4-5-10/h1-3,6H,4-5,10H2
InChIKey
AUCVZEYHEFAWHO-UHFFFAOYSA-N
Anwendung
3-Fluorophenethylamine may be used in the synthesis of:
- N-(3-florophenyl)ethylcaffeamide and its anti-inflammatory activity was evaluated
- N-{2-[(3-fluorophenyl)ethyl]}-2-methylpropanamide
- N-(3′-fluorophenyl)ethyl-4-azahexacyclo[5.4.1.02,6.03,10.05,9.08,11]dodecan-3-ol
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Skin Corr. 1B
Lagerklassenschlüssel
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flammpunkt (°F)
181.4 °F - closed cup
Flammpunkt (°C)
83 °C - closed cup
Persönliche Schutzausrüstung
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Jung-Chun Liao et al.
International journal of molecular sciences, 14(8), 15199-15211 (2013-07-28)
In this study, we evaluated the anti-inflammatory activity of one synthetic product, N-(3-Florophenyl)ethylcaffeamide (abbrev. FECA), by using animal model of λ-carrageenan-induced paw edema in mice. The anti-inflammatory mechanism of FECA was determined by measuring the levels of cyclooxygenase-2 (COX-2), nitric
Susumu Watanuki et al.
Chemical & pharmaceutical bulletin, 59(8), 1029-1037 (2011-08-02)
A series of 1-isopropyl-1,2,3,4-tetrahydroisoquinoline derivatives were synthesized and their bradycardic activities were evaluated in isolated guinea pig right atria. Structure-activity relationship studies revealed that the introduction of an appropriate substituent and its position on the 1,2,3,4-tetrahydroisoquinoline ring are essential for
Xiang Liu et al.
Medicinal chemistry (Shariqah (United Arab Emirates)), 1(1), 31-38 (2006-06-23)
Three new trishomocubane analogues based on the 4-azahexacyclo[5.4.1.0(2,6).0(3,10).0(5,9).0(8,11)] dodecane-3-ol skeleton have been synthesised and assessed for their affinities at both sigma-1 and sigma-2 receptors. The effect of various N-substitution on the polycyclic moiety was examined. All synthesised compounds displayed high
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.