90921
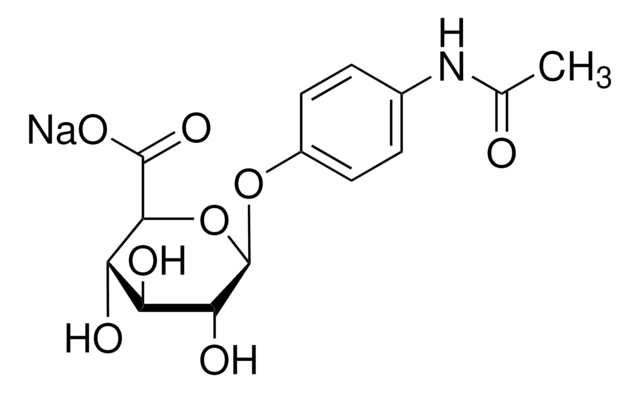
2-Acetamido-1,2-dideoxynojirimycin
≥98.0% (TLC)
Synonym(s):
2-Acetamido-1,2,5-trideoxy-1,5-imino-D-glucitol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H16N2O4
CAS Number:
Molecular Weight:
204.22
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
Assay
≥98.0% (TLC)
form
powder
storage temp.
2-8°C
SMILES string
CC(=O)N[C@H]1CN[C@H](CO)[C@@H](O)[C@@H]1O
InChI
1S/C8H16N2O4/c1-4(12)10-5-2-9-6(3-11)8(14)7(5)13/h5-9,11,13-14H,2-3H2,1H3,(H,10,12)/t5-,6+,7+,8+/m0/s1
InChI key
GBRAQQUMMCVTAV-LXGUWJNJSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2-Acetamido-1,2-dideoxynojirimycin (2-ADN) is used as an inhibitor (transition analogue) to identify, purify, differentiate and characterized N-acetylglucosaminidase(s) (GlcNAcase). 2-Acetamido-1,2-dideoxynojirimycin is used as a ligand for the affinity purification of N-acetylglucosaminidases.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Other Notes
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
H Böshagen et al.
Carbohydrate research, 164, 141-148 (1987-07-01)
The synthesis of 2-acetamido-1,2-dideoxynojirimycin (2-acetamido-1,2,5-tri-deoxy-1,5-imino-D-glucitol) by a double inversion procedure starting from 1-deoxynojirimycin is reported. The key intermediates were the selectively protected N-benzyl-1,5-dideoxy-1,5-imino-4,6-O-isopropylidene-D-mannitol, the triflate ester N-benzyl-3-O-benzyl-1,5-dideoxy-1,5-imino-4,6-O-isopropylidene-2-O- (tri-fluoromethylsulfonyl)-D-mannitol, and 2-azido-N-benzyl-3-O-benzyl-1,2,5-tri-deoxy-1,5-imino-4,6-O- isopropylidene-D-glucitol, readily obtained in a sequence from 1-deoxynojirimycin. Thus 1-deoxynojirimycin
B Woynarowska et al.
Anticancer research, 12(1), 161-166 (1992-01-01)
Human ovarian carcinoma (HOC) cell beta-N-acetylglucosaminidase (beta-NAG, EC 3.2.1.30) was found to be present in three isoenzymatic forms. All three forms were capable of degrading ECM. Therefore, inhibitors of beta-NAG were sought as potential anti-invasive agents. Two sugar analogs, 2-acetamido-2-deoxy-1,5-gluconolactone
G Legler et al.
Biochimica et biophysica acta, 1080(2), 89-95 (1991-10-25)
Two N-acetylglucosaminidases were isolated from bovine kidney with a three step procedure featuring affinity purification on 2-acetamido-1,2,5-trideoxy-1,5-iminoglucitol (2-acetamido-1,2-dideoxynojirimycin, II). The major isoenzyme, Hex A, is an alpha, beta hetero-dimer (57 and 52 kDa) with isoelectric points from pH 5.3 to
Yeon Kyu Kim et al.
Glycobiology, 19(3), 301-308 (2008-12-05)
Most insect cells have a simple N-glycosylation process and consequently paucimannosidic or simple core glycans predominate. Previously, we have shown that paucimannosidic N-glycan structures are dominant in Drosophila S2 cells. It has been proposed that beta-N-acetylglucosaminidase (GlcNAcase), a hexosaminidase in
G Gradnig et al.
Carbohydrate research, 287(1), 49-57 (1996-06-07)
6-Azido-1,3,4-tri-O-benzyl-6-deoxy-D-fructofuranose can be easily obtained in two steps from the known 6,6'-diazido-6,6'-dideoxysucrose (available in two steps from sucrose) and cyclized by controlled hydrogenation and concomitant intramolecular reductive amination to give 3,4,6-tri-O-benzyl-1,5-dideoxy-1,5-imino-D-mannitol, a partially protected derivative of 1-deoxymannojirimycin. After N-protection, position
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service