All Photos(3)
About This Item
Empirical Formula (Hill Notation):
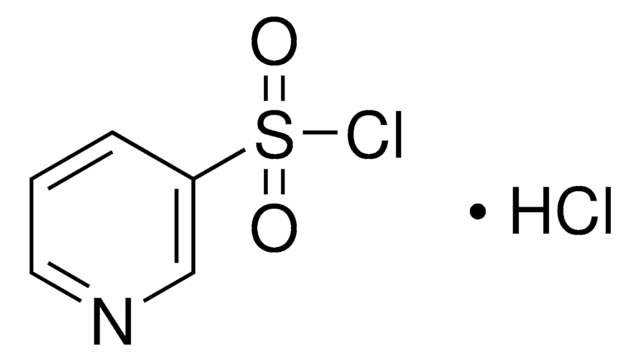
C9H6ClNO2S
CAS Number:
Molecular Weight:
227.67
Beilstein:
156347
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
126-129 °C (lit.)
SMILES string
ClS(=O)(=O)c1cccc2cccnc12
InChI
1S/C9H6ClNO2S/c10-14(12,13)8-5-1-3-7-4-2-6-11-9(7)8/h1-6H
InChI key
JUYUYCIJACTHMK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
J Borras et al.
Bioorganic & medicinal chemistry, 7(11), 2397-2406 (2000-01-13)
Reaction of 20 aromatic/heterocyclic sulfonamides containing a free amino, imino, hydrazino or hydroxyl group, with 8-quinoline-sulfonyl chloride afforded a series of water-soluble (as hydrochloride or triflate salts) compounds. The new derivatives were assayed as inhibitors of the zinc enzyme carbonic
Vijay K Agrawal et al.
European journal of medicinal chemistry, 39(7), 593-600 (2004-07-09)
Quantitative structure-activity-relationship (QSAR) study on aromatic/heterocyclic sulfonamides containing 8-quinoline-sulfonyl carbonic anhydrase (CA) inhibitors has been carried out topologically using first-order valence connectivity index ((1)chi(v)). Excellent results are obtained against all the three isozymes; CA I, II and IV of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service