All Photos(1)
About This Item
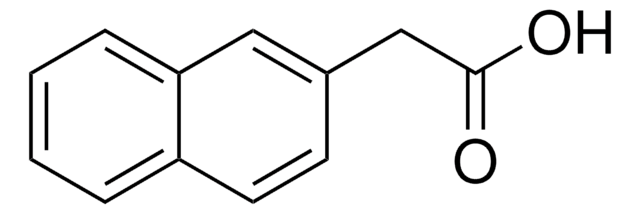
Linear Formula:
C10H7OCH2CO2H
CAS Number:
Molecular Weight:
202.21
Beilstein:
1074148
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
powder
mp
151-154 °C (lit.)
SMILES string
OC(=O)COc1ccc2ccccc2c1
InChI
1S/C12H10O3/c13-12(14)8-15-11-6-5-9-3-1-2-4-10(9)7-11/h1-7H,8H2,(H,13,14)
InChI key
RZCJYMOBWVJQGV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
M Illangasekare et al.
Science (New York, N.Y.), 267(5198), 643-647 (1995-02-03)
An RNA has been selected that rapidly aminoacylates its 2'(3') terminus when provided with phenylalanyl-adenosine monophosphate. That is, the RNA accelerates the same aminoacyl group transfer catalyzed by protein aminoacyl-transfer RNA synthetases. The best characterized RNA reaction requires both Mg2+
Poonam Piplani et al.
Medicinal chemistry (Shariqah (United Arab Emirates)), 9(3), 371-378 (2012-08-28)
The present paper describes the design and synthesis of a series of some 2-naphthyloxy derivatives with their antiamnesic activity using mice as the animal model and piracetam as the reference drug. All the synthesized compounds were characterized by spectroscopic techniques
D Hössel et al.
Plant biology (Stuttgart, Germany), 7(1), 41-48 (2005-01-25)
A study of transport and action of synthetic auxin analogues can help to identify transporters and receptors of this plant hormone. Both aspects--transportability and action on growth--were tested with 2-naphthoxyacetic acid (2-NOA) and compared across several plant species. 2-NOA stimulates
V Gökmen et al.
Journal of chromatography. A, 798(1-2), 167-171 (1998-05-02)
An alternative high-performance liquid chromatographic method for the determination of beta-naphthoxyacetic acid (BNOA) in tomatoes is described. BNOA was extracted from tomatoes with acetone-dichloromethane (2:1). The extract was cleaned up by Bio-Beads S-X3 gel-permeation chromatography and by partitioning. A reversed-phase
Residue analysis of beta-naphthoxyacetic acid and beta-naphthol on field-sprayed tomatoes by high-pressure liquid chromatography.
T E Archer et al.
Journal of agricultural and food chemistry, 28(4), 877-880 (1980-07-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service