B2761
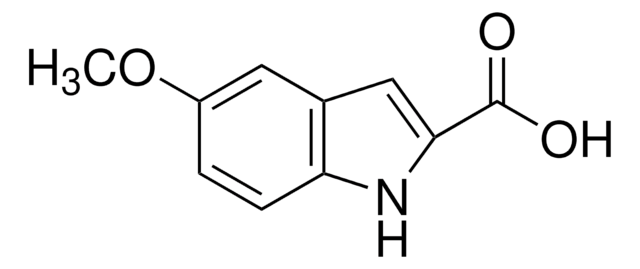
5-Bromoindole-2-carboxylic acid
98%
Synonym(s):
NSC 73384
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C9H6NO2Br
CAS Number:
Molecular Weight:
240.05
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
powder
storage temp.
−20°C
SMILES string
OC(=O)c1cc2cc(Br)ccc2[nH]1
InChI
1S/C9H6BrNO2/c10-6-1-2-7-5(3-6)4-8(11-7)9(12)13/h1-4,11H,(H,12,13)
InChI key
YAULOOYNCJDPPU-UHFFFAOYSA-N
Application
Reactant involved in studies of biologically active molecules including:
- Discovery of indole inhibitors of MMP-13 for treatment of arthritic diseases
- Synthesis of indolyl ethanones as indoleamine 2,3-dioxygenase inhibitors
- cis-Diaminocyclohexane derivatives prepared for use as factor Xa inhibitors
- Synthesis of tubulin polymerization inhibitors and cancer cell growth inhibitors
- Preparation of dual PPARγ/δ agonists
- Synthesis of chemical probes to examine the role of hFPRL1 receptor in inflammation
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Shahrbanoo Jahangir et al.
Stem cell research & therapy, 11(1), 436-436 (2020-10-11)
Mesenchymal stem cells are a promising cell source for chondrogenic differentiation and have been widely used in several preclinical and clinical studies. However, they are prone to an unwanted differentiation process towards hypertrophy that limits their therapeutic efficacy. Matrix metallopeptidase
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service