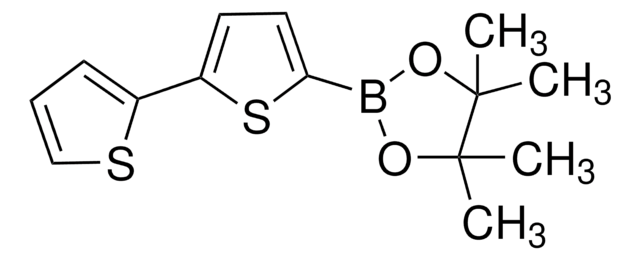
647020
2,2′-Bithiophene-5,5′-diboronic acid bis(pinacol) ester
97%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C20H28B2O4S2
CAS Number:
Molecular Weight:
418.19
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
208-212 °C (lit.)
SMILES string
CC1(C)OB(OC1(C)C)c2ccc(s2)-c3ccc(s3)B4OC(C)(C)C(C)(C)O4
InChI
1S/C20H28B2O4S2/c1-17(2)18(3,4)24-21(23-17)15-11-9-13(27-15)14-10-12-16(28-14)22-25-19(5,6)20(7,8)26-22/h9-12H,1-8H3
InChI key
XWWXVHGWYCXJCJ-UHFFFAOYSA-N
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Taichi Ikeda et al.
Nanoscale, 8(30), 14673-14681 (2016-07-21)
Oligomers of tetra(ethylene glycol)-disubstituted phenyl-capped bithiophene (Ph2TPh) linked by catechol and resorcinol were prepared. Catechol and resorcinol link the monomers via the ortho- and meta-positions of the benzene ring, respectively, and function as turning points in the folding process of
Articles
Oligothiophenes are important organic electronic materials which can be produced using synthetic intermediates and Suzuki coupling.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)