All Photos(1)
About This Item
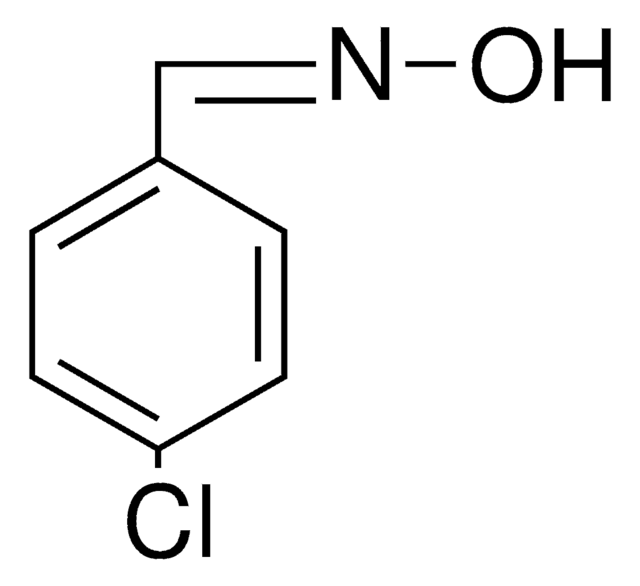
Linear Formula:
ClC6H4CH=NOH
CAS Number:
Molecular Weight:
155.58
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
73-76 °C (lit.)
SMILES string
O\N=C\c1ccccc1Cl
InChI
1S/C7H6ClNO/c8-7-4-2-1-3-6(7)5-9-10/h1-5,10H/b9-5+
InChI key
FZIVKDWRLLMSEJ-WEVVVXLNSA-N
Related Categories
General description
2-Chlorobenzaldehyde oxime is also known as o-chlorobenzaldehyde oxime. It can be synthesized by reacting 2-chlorobenzaldehyde and hydroxylamine hydrochloride.
Application
2-Chlorobenzaldehyde oxime may be used in the preparation of:
- 2-chlorobenzaldehyde under different reaction conditions
- methyl 3-(2-chlorophenyl)-5-[1-(4-methoxybenzyloxy)-ethyl]isoxazole-4-carboxylate
- dimethyl 3-(2-chlorophenyl)isoxazole-4,5-dicarboxylate
- [3-(2-chlorophenyl)isoxazol-5-yl]methanol
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A mild and selective method for the conversion of oximes into ketones and aldehydes by the use of N-bromophthalimide.
Khazaei A, et al.
J. Chem. Res. (M), 2004(10), 695-696 (2004)
Hypervalent iodine mediated synthesis of di-and tri-substituted isoxazoles via [3+2] cycloaddition of nitrile oxides.
Singhal A, et al.
Tetrahedron, 57(7), 719-722 (2016)
Solid-phase synthesis of 5-isoxazol-4-yl-[1,2,4] oxadiazoles.
Quan C and Kurth M.
The Journal of Organic Chemistry, 69(5), 1470-1474 (2004)
Microwave-assisted chemoselective cleavage of oximes to their corresponding carbonyl compounds using 1, 3-dichloro-5, 5-dimethyl-hydantoin (DCDMH) as a new Deoximating reagent.
Khazaei A and Manesh AA.
Synthesis, 2005(12), 1929-1931 (2005)
Amberlyst 15 supported nitrosonium ion as an efficient reagent for regeneration of carbonyl compounds from oximes, hydrazones and semicarbazones.
Lakouraj MM, et al.
Reactive functional Polymers, 66(9), 910-915 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service