All Photos(1)
About This Item
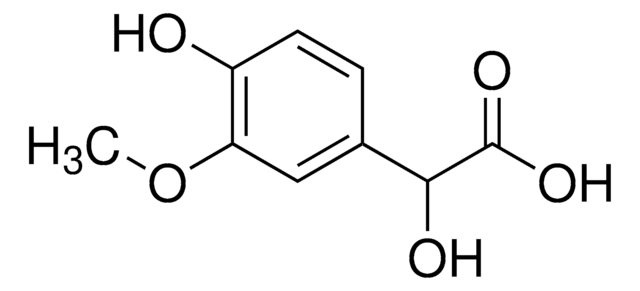
Linear Formula:
CH3OC6H4CH(OH)CO2H
CAS Number:
Molecular Weight:
182.17
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
108-111 °C (lit.)
SMILES string
COc1ccc(cc1)C(O)C(O)=O
InChI
1S/C9H10O4/c1-13-7-4-2-6(3-5-7)8(10)9(11)12/h2-5,8,10H,1H3,(H,11,12)
InChI key
ITECRQOOEQWFPE-UHFFFAOYSA-N
General description
Chiral separation of the enantiomers of 4-methoxymandelic acid has been reported by a new liquid chromatographic method. The mechanism of veratryl alcohol-mediated oxidation of 4-methoxymandelic acid by lignin peroxidase has been studied by kinetic methods.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Radical cation cofactors in lignin peroxidase catalysis.
P J Harvey et al.
Biochemical Society transactions, 23(2), 262-267 (1995-05-01)
Jie Zhou et al.
Journal of Zhejiang University. Science. B, 14(7), 615-620 (2013-07-05)
A new liquid chromatographic method has been developed for the chiral separation of the enantiomers of mandelic acid and their derivatives 2-chloromandelic acid, 4-hydroxymandelic acid, 4-methoxymandelic acid, and 3,4,5-trismethoxymandelic acid. The enantiomers were separated by a CHIRALPAK(®) IC (250 mm×4.6
Ida Fejős et al.
Journal of chromatography. A, 1467, 454-462 (2016-07-28)
The enantioselectivity of neutral single-isomer synthetic precursors of sulfated-β-cyclodextrins was studied. Four neutral single-isomer cyclodextrins substituted on the secondary side with acetyl and/or methyl functional groups, heptakis(2-O-methyl-3,6-dihydroxy)-β-cyclodextrin (HM-β-CD), heptakis(2,3-di-O-acetyl-6-hydroxy)-β-cyclodextrin (HDA-β-CD), heptakis(2,3-di-O-methyl-6-hydroxy)-β-cyclodextrin (HDM-β-CD), heptakis(2-O-methyl-3-O-acetyl-6-hydroxy)-β-cyclodextrin (HMA-β-CD), and their sulfated analogs the negatively
K Valli et al.
Biochemistry, 29(37), 8535-8539 (1990-09-18)
Lignin peroxidase (LiP), an extracellular heme enzyme from the lignin-degrading fungus Phanerochaete chrysosporium, catalyzes the H2O2-dependent oxidation of a variety of nonphenolic lignin model compounds. The oxidation of monomethoxylated lignin model compounds, such as anisyl alcohol (AA), and the role
L P Candeias et al.
The Journal of biological chemistry, 270(28), 16745-16748 (1995-07-14)
The formation and decay of veratryl alcohol radical cation upon oxidation of veratryl alcohol by thallium (II) ions was studied by pulse radiolysis with spectrophotometric and conductometric detection. In aqueous solution at pH 3 the radical cation decays by a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service