35601
PCB No 28
analytical standard
Synonym(s):
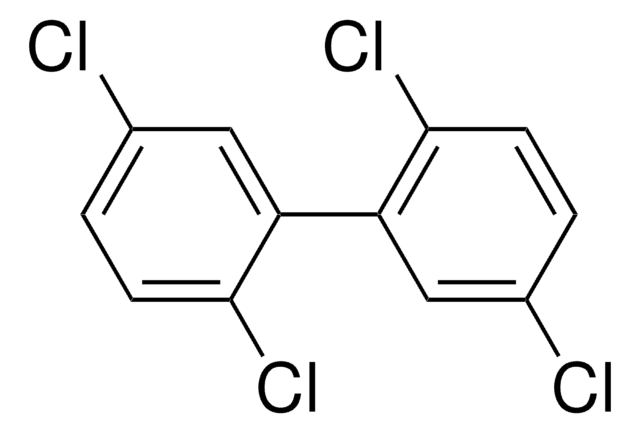
2,4,4′-Trichlorobiphenyl, 2,4,4′-PCB
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C12H7Cl3
CAS Number:
Molecular Weight:
257.54
Beilstein:
1956846
Ballschmiter Number:
28
EC Number:
MDL number:
UNSPSC Code:
41116107
PubChem Substance ID:
NACRES:
NA.24
Recommended Products
grade
analytical standard
Quality Level
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
environmental
format
neat
SMILES string
Clc1ccc(cc1)-c2ccc(Cl)cc2Cl
InChI
1S/C12H7Cl3/c13-9-3-1-8(2-4-9)11-6-5-10(14)7-12(11)15/h1-7H
InChI key
BZTYNSQSZHARAZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Polychlorinated biphenyls (PCBs) are groups of chemical compounds which are persistent in the environment and are considered highly toxic to humans and animals. PCB No 28 may be used for environmental analysis and precise quality control of food and feed.
Application
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
Recommended products
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - STOT RE 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Li Xu et al.
Huan jing ke xue= Huanjing kexue, 31(1), 255-259 (2010-03-25)
The capability of Rhizobium meliloti on degrading 2,4,4'-trichlorobiphenyl and 18 kinds of polychlorinated biphenyl congener mixtures was studied by shaking flask experiment. The results showed that the degradation capability of Rhizobium meliloti to 2, 4, 4'-trichlorobiphenyl increased gradually with the
Xiang-Hui Sun et al.
Huan jing ke xue= Huanjing kexue, 31(10), 2327-2330 (2011-01-15)
Sorption behaviors of PCB28 to phosphatidylcholine (PC)and triglyceride (TG) were studied. Results showed that sorption equilibrium could be achieved in 8 h for PCB28, and the sorption amount on PC was higher than that of TG when the initial PCB28
Stuart Harrad et al.
Journal of environmental monitoring : JEM, 9(4), 314-318 (2007-04-06)
The environmentally prevalent polybrominated diphenyl ether (PBDE) #47 and polychlorinated biphenyls (PCBs) #28 and #118 were challenged for 24 hours with a novel biomass-supported Pd catalyst (Bio-Pd(0)). Analysis of the products via GC-MS revealed the Bio-Pd(0) to cause the challenged
A J Lambo et al.
Journal of applied microbiology, 102(5), 1318-1329 (2007-04-24)
To determine the extent and pattern of degradation of polychlorinated biphenyls (PCBs) in Aroclor 1232 at 5 degrees C by a psychrotolerant bacterium, and to confirm the formation of intermediates of PCB metabolism at low temperature using 2,4,4'-trichlorobiphenyl (2,4,4'-TCB). 10
Leticia Gómez-Gil et al.
Journal of bacteriology, 189(15), 5705-5715 (2007-05-29)
Biphenyl dioxygenase (BPDO) catalyzes the aerobic transformation of biphenyl and various polychlorinated biphenyls (PCBs). In three different assays, BPDO(B356) from Pandoraea pnomenusa B-356 was a more potent PCB-degrading enzyme than BPDO(LB400) from Burkholderia xenovorans LB400 (75% amino acid sequence identity)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service