20025
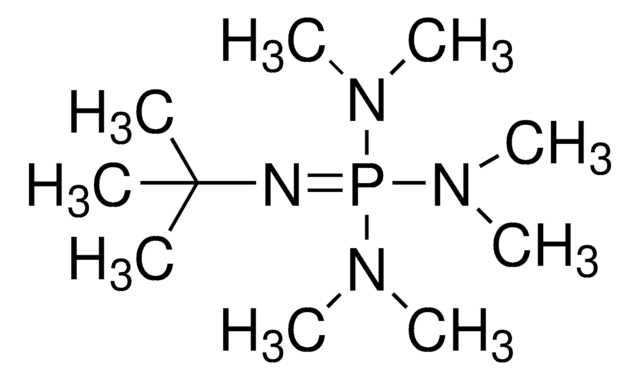
2-tert-Butylimino-2-diethylamino-1,3-dimethylperhydro-1,3,2-diazaphosphorine
purum, ≥98.0% (GC)
Synonym(s):
BEMP
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C13H31N4P
CAS Number:
Molecular Weight:
274.39
Beilstein:
5534901
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
purum
Quality Level
Assay
≥98.0% (GC)
form
liquid
refractive index
n20/D 1.477 (lit.)
n20/D 1.477
bp
74 °C/0.03 mmHg (lit.)
density
0.948 g/mL at 25 °C (lit.)
functional group
amine
SMILES string
CCN(CC)P1(=NC(C)(C)C)N(C)CCCN1C
InChI
1S/C13H31N4P/c1-8-17(9-2)18(14-13(3,4)5)15(6)11-10-12-16(18)7/h8-12H2,1-7H3
InChI key
VSCBATMPTLKTOV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2-tert-Butylimino-2-diethylamino-1,3-dimethylperhydro-1,3,2-diazaphosphorine (BEMP) is a phosphazene base. BEMP participates in the mild base catalyzed nucleophilic ring opening of N-sulfonyl aziridines. BEMP supported on polystyrene (PS-BEMP) has been reported as an efficient catalyst for the ring-opening of epoxides with phenols. BEMP is about 2000 times more basic and also much more sterically hindered than DBU (l,8-diazabicyclo[5.4.0]undecene).
Application
2-tert-Butylimino-2-diethylamino-1,3-dimethylperhydro-1,3,2-diazaphosphorine (BEMP), a organic phosphorine base may be used in the following studies:
- Synthesis of a 2,3-dihydrobenzo[1,4]dioxepin-5-one.
- As catalyst in the alkylation reactions of carbon acids.
- As organocatalyst in the controlled "immortal" ring-opening polymerization (iROP) of six-membered cyclic carbonates.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Marion Helou et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 16(46), 13805-13813 (2010-10-15)
Six-membered cyclic carbonates, namely trimethylene carbonate (TMC), 3,3-dimethoxytrimethylene carbonate (DMTMC) and 3-benzyloxytrimethylene carbonate (BTMC), undergo controlled "immortal" ring-opening polymerization (iROP) under mild conditions (bulk, 60-150 °C), by using organocatalysts, including an amine [4-N,N-dimethylaminopyridine (DMAP)], a guanidine [1,5,7-triazabicyclo-[4.4.0]dec-5-ene (TBD)], or a
Sambasivarao Kotha et al.
Bioorganic & medicinal chemistry letters, 12(7), 1113-1115 (2002-03-23)
First synthesis of a macrocylic cyclophane-based unusual alpha-amino acid derivative 11 by coupling of ethyl isocyanoacetate with 1,2-bis(4-bromomethylphenyl)ethane under phase-transfer catalysis (PTC) conditions. Phosphazene base such as 2-tert-butylimino-2-diethylamino-1,3-dimethylperhydro-1,3,2-diazaphosphorine (BEMP) is useful to improve the yield of cyclophane derivative without high
Thomas A Moss et al.
Chemical communications (Cambridge, England), (21)(21), 2474-2476 (2008-05-21)
N-Mesitylene sulfonyl and N-tosyl aziridines have been identified as effective electrophiles in alkylation reactions of carbon acids catalyzed by the organic phosphorine base BEMP; yields of up to 99% for a range of pro-nucleophiles under mild reaction conditions are reported.
2-tert-Butylimino-2-diethylamino-1, 3-dimethylperhydro-1, 3, 2-diazaphosphorine Supported on Polystyrene (PS-BEMP) as an Efficient Recoverable and Reusable Catalyst for the Phenolysis of Epoxides under Solvent-Free Conditions.
Zvagulis A, et al.
Advanced Synthesis & Catalysis, 352(14-15), 2489-2496 (2010)
Ke Wen et al.
The Journal of organic chemistry, 67(22), 7887-7889 (2002-10-26)
A short and efficient synthesis of 2'-O-methoxyethylguanosine (8) is described. Central to this strategy is the development of a novel silicon-based protecting group (MDPSCl(2), 2) used to protect the 3',5'-hydroxyl groups of the ribose. Silylation of guanosine with 2 proceeded
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)
![1,5,7-Triazabicyclo[4.4.0]dec-5-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/171/446/333d560c-cff6-4958-b489-5acfb3057cce/640/333d560c-cff6-4958-b489-5acfb3057cce.png)
![7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/237/769/028967ef-ca63-4f22-acc9-68f135a43b9a/640/028967ef-ca63-4f22-acc9-68f135a43b9a.png)



